Human CD83-targeted chimeric antigen receptor T cells prevent and treat graft-versus-host disease
- PMID: 32437331
- PMCID: PMC7456225
- DOI: 10.1172/JCI135754
Human CD83-targeted chimeric antigen receptor T cells prevent and treat graft-versus-host disease
Abstract
Graft-versus-host disease (GVHD) remains an important cause of morbidity and mortality after allogeneic hematopoietic cell transplantation (allo-HCT). For decades, GVHD prophylaxis has included calcineurin inhibitors, despite their incomplete efficacy and impairment of graft-versus-leukemia (GVL). Distinct from pharmacologic immune suppression, we have developed what we believe is a novel, human CD83-targeted chimeric antigen receptor (CAR) T cell for GVHD prevention. CD83 is expressed on allo-activated conventional CD4+ T cells (Tconvs) and proinflammatory dendritic cells (DCs), which are both implicated in GVHD pathogenesis. Human CD83 CAR T cells eradicate pathogenic CD83+ target cells, substantially increase the ratio of regulatory T cells (Tregs) to allo-activated Tconvs, and provide durable prevention of xenogeneic GVHD. CD83 CAR T cells are also capable of treating xenogeneic GVHD. We show that human acute myeloid leukemia (AML) expresses CD83 and that myeloid leukemia cell lines are readily killed by CD83 CAR T cells. Human CD83 CAR T cells are a promising cell-based approach to preventing 2 critical complications of allo-HCT - GVHD and relapse. Thus, the use of human CD83 CAR T cells for GVHD prevention and treatment, as well as for targeting CD83+ AML, warrants clinical investigation.
Keywords: Cancer immunotherapy; Oncology; Stem cell transplantation; T cells; Transplantation.
Conflict of interest statement
Figures
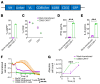



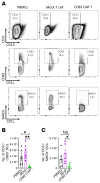

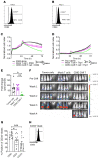
References
-
- Powles RL, Barrett AJ, Clink H, Kay HE, Sloane J, McElwain TJ. Cyclosporin A for the treatment of graft-versus-host disease in man. Lancet. 1978;2(8104-5):1327–1331. - PubMed
-
- Storb R, et al. Marrow transplantation for severe aplastic anemia: methotrexate alone compared with a combination of methotrexate and cyclosporine for prevention of acute graft-versus-host disease. Blood. 1986;68(1):119–1251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL133823/HL/NHLBI NIH HHS/United States
- P30 CA077598/CA/NCI NIH HHS/United States
- R01 HL118979/HL/NHLBI NIH HHS/United States
- R01 HL126530/HL/NHLBI NIH HHS/United States
- R01 HL147324/HL/NHLBI NIH HHS/United States
- R37 AI034495/AI/NIAID NIH HHS/United States
- P30 CA076292/CA/NCI NIH HHS/United States
- R01 HL155114/HL/NHLBI NIH HHS/United States
- R01 HL056067/HL/NHLBI NIH HHS/United States
- K08 HL116547/HL/NHLBI NIH HHS/United States
- P01 CA142106/CA/NCI NIH HHS/United States
- K12 CA090625/CA/NCI NIH HHS/United States
- P01 CA065493/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

