THP-1 macrophage cholesterol efflux is impaired by palmitoleate through Akt activation
- PMID: 32437392
- PMCID: PMC7241781
- DOI: 10.1371/journal.pone.0233180
THP-1 macrophage cholesterol efflux is impaired by palmitoleate through Akt activation
Abstract
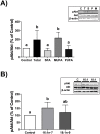
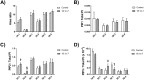
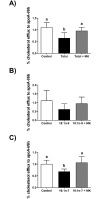
Lipoprotein lipase (LPL) is upregulated in atherosclerotic lesions and it may promote the progression of atherosclerosis, but the mechanisms behind this process are not completely understood. We previously showed that the phosphorylation of Akt within THP-1 macrophages is increased in response to the lipid hydrolysis products generated by LPL from total lipoproteins. Notably, the free fatty acid (FFA) component was responsible for this effect. In the present study, we aimed to reveal more detail as to how the FFA component may affect Akt signalling. We show that the phosphorylation of Akt within THP-1 macrophages increases with total FFA concentration and that phosphorylation is elevated up to 18 hours. We further show that specifically the palmitoleate component of the total FFA affects Akt phosphorylation. This is tied with changes to the levels of select molecular species of phosphoinositides. We further show that the total FFA component, and specifically palmitoleate, reduces apolipoprotein A-I-mediated cholesterol efflux, and that the reduction can be reversed in the presence of the Akt inhibitor MK-2206. Overall, our data support a negative role for the FFA component of lipoprotein hydrolysis products generated by LPL, by impairing macrophage cholesterol efflux via Akt activation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- McCoy MG, Sun GS, Marchadier D, Maugeais C, Glick JM, & Rader DJ. Characterization of the lipolytic activity of endothelial lipase. J Lipid Res. 2002;43(6):921–929. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

