Interferon regulatory factor 7 mediates obesity-associated MCP-1 transcription
- PMID: 32437400
- PMCID: PMC7241760
- DOI: 10.1371/journal.pone.0233390
Interferon regulatory factor 7 mediates obesity-associated MCP-1 transcription
Abstract
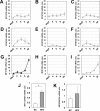
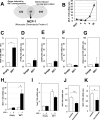
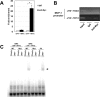
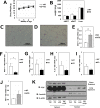
Hypertrophy, associated with adipocyte dysfunction, causes increased pro-inflammatory adipokine, and abnormal glucose and lipid metabolism, leading to insulin resistance and obesity-related-health problems. By combining DNA microarray and genomic data analyses to predict DNA binding motifs, we identified the transcription factor Interferon Regulatory Factor 7 (IRF7) as a possible regulator of genes related to adipocyte hypertrophy. To investigate the role of IRF7 in adipocytes, we examined gene expression patterns in 3T3-L1 cells infected with a retrovirus carrying the IRF7 gene and found that enforced IRF7 expression induced the expression of monocyte chemoattractant protein-1 (MCP-1), a key initial adipokine in the chronic inflammation of obesity. CRISPR/Cas9 mediated-suppression of IRF7 significantly reduced MCP-1 mRNA. Luciferase assays, chromatin immunoprecipitation PCR analysis and gel shift assay showed that IRF7 transactivates the MCP-1 gene by binding to its proximal Interferon Stimulation Response Element (ISRE), a putative IRF7 binding motif. IRF7 knockout mice exhibited lower expression of MCP-1 in epidydimal white adipose tissue under high-fat feeding conditions, suggesting the transcription factor is physiologically important for inducing MCP-1. Taken together, our results suggest that IRF7 transactivates MCP-1 mRNA in adipocytes, and it may be involved in the adipose tissue inflammation associated with obesity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

