Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial
- PMID: 32437507
- PMCID: PMC7243167
- DOI: 10.1001/jamaoncol.2020.1024
Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial
Abstract
Importance: Clinical outcomes for glioblastoma remain poor. Treatment with immune checkpoint blockade has shown benefits in many cancer types. To our knowledge, data from a randomized phase 3 clinical trial evaluating a programmed death-1 (PD-1) inhibitor therapy for glioblastoma have not been reported.
Objective: To determine whether single-agent PD-1 blockade with nivolumab improves survival in patients with recurrent glioblastoma compared with bevacizumab.
Design, setting, and participants: In this open-label, randomized, phase 3 clinical trial, 439 patients with glioblastoma at first recurrence following standard radiation and temozolomide therapy were enrolled, and 369 were randomized. Patients were enrolled between September 2014 and May 2015. The median follow-up was 9.5 months at data cutoff of January 20, 2017. The study included 57 multicenter, multinational clinical sites.
Interventions: Patients were randomized 1:1 to nivolumab 3 mg/kg or bevacizumab 10 mg/kg every 2 weeks until confirmed disease progression, unacceptable toxic effects, or death.
Main outcomes and measures: The primary end point was overall survival (OS).
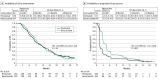
Results: A total of 369 patients were randomized to nivolumab (n = 184) or bevacizumab (n = 185). The MGMT promoter was methylated in 23.4% (43/184; nivolumab) and 22.7% (42/185; bevacizumab), unmethylated in 32.1% (59/184; nivolumab) and 36.2% (67/185; bevacizumab), and not reported in remaining patients. At median follow-up of 9.5 months, median OS (mOS) was comparable between groups: nivolumab, 9.8 months (95% CI, 8.2-11.8); bevacizumab, 10.0 months (95% CI, 9.0-11.8); HR, 1.04 (95% CI, 0.83-1.30); P = .76. The 12-month OS was 42% in both groups. The objective response rate was higher with bevacizumab (23.1%; 95% CI, 16.7%-30.5%) vs nivolumab (7.8%; 95% CI, 4.1%-13.3%). Grade 3/4 treatment-related adverse events (TRAEs) were similar between groups (nivolumab, 33/182 [18.1%]; bevacizumab, 25/165 [15.2%]), with no unexpected neurological TRAEs or deaths due to TRAEs.
Conclusions and relevance: Although the primary end point was not met in this randomized clinical trial, mOS was comparable between nivolumab and bevacizumab in the overall patient population with recurrent glioblastoma. The safety profile of nivolumab in patients with glioblastoma was consistent with that in other tumor types.
Trial registration: ClinicalTrials.gov Identifier: NCT02017717.
Conflict of interest statement
Figures


Comment in
-
Results From the CheckMate 143 Clinical Trial: Stalemate or New Game Strategy for Glioblastoma Immunotherapy?JAMA Oncol. 2020 Jul 1;6(7):987-989. doi: 10.1001/jamaoncol.2020.0857. JAMA Oncol. 2020. PMID: 32437542 No abstract available.
-
Appropriate Analysis of Duration of Response Data in Cancer Trials.JAMA Oncol. 2020 Dec 1;6(12):1978. doi: 10.1001/jamaoncol.2020.4657. JAMA Oncol. 2020. PMID: 33030502 Free PMC article. No abstract available.
-
Appropriate Analysis of Duration of Response Data in Cancer Trials-Reply.JAMA Oncol. 2020 Dec 1;6(12):1978-1979. doi: 10.1001/jamaoncol.2020.4664. JAMA Oncol. 2020. PMID: 33030510 No abstract available.
References
-
- Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466. doi:10.1016/S1470-2045(09)70025-7 - DOI - PubMed
-
- Weller M, van den Bent M, Tonn JC, et al. ; European Association for Neuro-Oncology (EANO) Task Force on Gliomas . European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315-e329. doi:10.1016/S1470-2045(17)30194-8 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

