Distinctive binding properties of human monoclonal LGI1 autoantibodies determine pathogenic mechanisms
- PMID: 32437528
- PMCID: PMC7296845
- DOI: 10.1093/brain/awaa104
Distinctive binding properties of human monoclonal LGI1 autoantibodies determine pathogenic mechanisms
Abstract
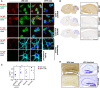
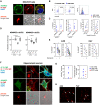
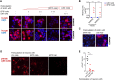
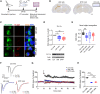
Autoantibodies against leucine-rich glioma inactivated 1 (LGI1) are found in patients with limbic encephalitis and focal seizures. Here, we generate patient-derived monoclonal antibodies (mAbs) against LGI1. We explore their sequences and binding characteristics, plus their pathogenic potential using transfected HEK293T cells, rodent neuronal preparations, and behavioural and electrophysiological assessments in vivo after mAb injections into the rodent hippocampus. In live cell-based assays, LGI1 epitope recognition was examined with patient sera (n = 31), CSFs (n = 11), longitudinal serum samples (n = 15), and using mAbs (n = 14) generated from peripheral B cells of two patients. All sera and 9/11 CSFs bound both the leucine-rich repeat (LRR) and the epitempin repeat (EPTP) domains of LGI1, with stable ratios of LRR:EPTP antibody levels over time. By contrast, the mAbs derived from both patients recognized either the LRR or EPTP domain. mAbs against both domain specificities showed varied binding strengths, and marked genetic heterogeneity, with high mutation frequencies. LRR-specific mAbs recognized LGI1 docked to its interaction partners, ADAM22 and ADAM23, bound to rodent brain sections, and induced internalization of the LGI1-ADAM22/23 complex in both HEK293T cells and live hippocampal neurons. By contrast, few EPTP-specific mAbs bound to rodent brain sections or ADAM22/23-docked LGI1, but all inhibited the docking of LGI1 to ADAM22/23. After intrahippocampal injection, and by contrast to the LRR-directed mAbs, the EPTP-directed mAbs showed far less avid binding to brain tissue and were consistently detected in the serum. Post-injection, both domain-specific mAbs abrogated long-term potentiation induction, and LRR-directed antibodies with higher binding strengths induced memory impairment. Taken together, two largely dichotomous populations of LGI1 mAbs with distinct domain binding characteristics exist in the affinity matured peripheral autoantigen-specific memory pools of individuals, both of which have pathogenic potential. In human autoantibody-mediated diseases, the detailed characterization of patient mAbs provides a valuable method to dissect the molecular mechanisms within polyclonal populations.
Keywords: LGI1; antibody; autoimmune; encephalitis; immunology.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


Comment in
-
Synaptic autoimmunity: new insights into LGI1 antibody-mediated neuronal dysfunction.Brain. 2020 Jun 1;143(6):1622-1625. doi: 10.1093/brain/awaa153. Brain. 2020. PMID: 32543694 No abstract available.
References
-
- Finke C, Prüss H, Heine J, Reuter S, Kopp UA, Wegner F, et al. Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine-rich, glioma-inactivated 1 antibodies. JAMA Neurol 2017; 74: 50–9. - PubMed
-
- Fukata Y, Adesnik H, Iwanaga T, Bredt DS, Nicoll RA, Fukata M.. Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science 2006; 313: 1792–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

