Clinical and experimental insight into pathophysiology, comorbidity and therapy of absence seizures
- PMID: 32437558
- PMCID: PMC7447525
- DOI: 10.1093/brain/awaa072
Clinical and experimental insight into pathophysiology, comorbidity and therapy of absence seizures
Abstract
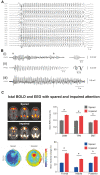
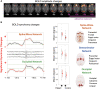
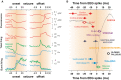
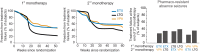
Absence seizures in children and teenagers are generally considered relatively benign because of their non-convulsive nature and the large incidence of remittance in early adulthood. Recent studies, however, show that 30% of children with absence seizures are pharmaco-resistant and 60% are affected by severe neuropsychiatric comorbid conditions, including impairments in attention, cognition, memory and mood. In particular, attention deficits can be detected before the epilepsy diagnosis, may persist even when seizures are pharmacologically controlled and are aggravated by valproic acid monotherapy. New functional MRI-magnetoencephalography and functional MRI-EEG studies provide conclusive evidence that changes in blood oxygenation level-dependent signal amplitude and frequency in children with absence seizures can be detected in specific cortical networks at least 1 min before the start of a seizure, spike-wave discharges are not generalized at seizure onset and abnormal cortical network states remain during interictal periods. From a neurobiological perspective, recent electrical recordings and imaging of large neuronal ensembles with single-cell resolution in non-anaesthetized models show that, in contrast to the predominant opinion, cortical mechanisms, rather than an exclusively thalamic rhythmogenesis, are key in driving seizure ictogenesis and determining spike-wave frequency. Though synchronous ictal firing characterizes cortical and thalamic activity at the population level, individual cortico-thalamic and thalamocortical neurons are sparsely recruited to successive seizures and consecutive paroxysmal cycles within a seizure. New evidence strengthens previous findings on the essential role for basal ganglia networks in absence seizures, in particular the ictal increase in firing of substantia nigra GABAergic neurons. Thus, a key feature of thalamic ictogenesis is the powerful increase in the inhibition of thalamocortical neurons that originates at least from two sources, substantia nigra and thalamic reticular nucleus. This undoubtedly provides a major contribution to the ictal decrease in total firing and the ictal increase of T-type calcium channel-mediated burst firing of thalamocortical neurons, though the latter is not essential for seizure expression. Moreover, in some children and animal models with absence seizures, the ictal increase in thalamic inhibition is enhanced by the loss-of-function of the astrocytic GABA transporter GAT-1 that does not necessarily derive from a mutation in its gene. Together, these novel clinical and experimental findings bring about paradigm-shifting views of our understanding of absence seizures and demand careful choice of initial monotherapy and continuous neuropsychiatric evaluation of affected children. These issues are discussed here to focus future clinical and experimental research and help to identify novel therapeutic targets for treating both absence seizures and their comorbidities.
Keywords: anti-absence drugs; attention deficits; basal ganglia; cortico-thalamo-cortical loop; limbic system.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures




References
-
- Adamantidis AR, Gutierrez Herrera C, Gent TC.. Oscillating circuitries in the sleeping brain. Nat Rev Neurosci 2019; 20: 746–62. - PubMed
-
- Aghakhani Y, Bagshaw AP, Bénar CG, Hawco C, Andermann F, Dubeau F, et al.fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain 2004; 127: 1127–44. - PubMed
-
- Aker RG, Yananli HR, Gurbanova AA, Ozkaynakci AE, Ates N, Luijtelaar G, et al.Amygdala kindling in the WAG/Rij rat model of absence epilepsy. Epilepsia 2006; 47: 33–40. - PubMed
-
- Aldenkamp AP, Arends J, Overweg-Plandsoen TC, van Bronswijk KC, Schyns-Soeterboek A, Linden I, et al.Acute cognitive effects of nonconvulsive difficult-to-detect epileptic seizures and epileptiform electroencephalographic discharges. J Child Neurol 2001; 16: 119–23. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

