Achieving Site-Selectivity for C-H Activation Processes Based on Distance and Geometry: A Carpenter's Approach
- PMID: 32437604
- PMCID: PMC7485751
- DOI: 10.1021/jacs.0c04074
Achieving Site-Selectivity for C-H Activation Processes Based on Distance and Geometry: A Carpenter's Approach
Abstract
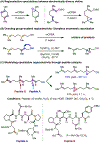
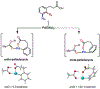
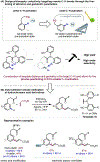
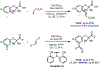
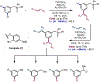
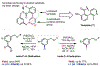
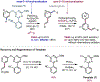
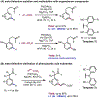
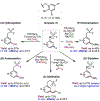
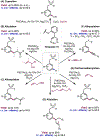
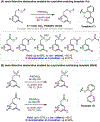
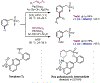
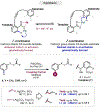
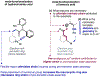
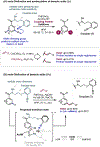
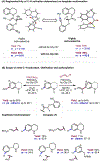
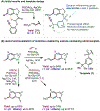
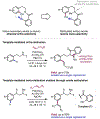
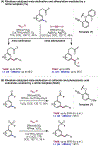
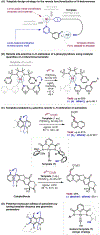
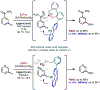
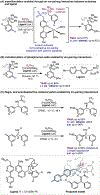
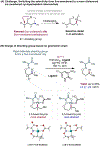
The ability to differentiate between highly similar C-H bonds in a given molecule remains a fundamental challenge in organic chemistry. In particular, the lack of sufficient steric and electronic differences between C-H bonds located distal to functional groups has prevented the development of site-selective catalysts with broad scope. An emerging approach to circumvent this obstacle is to utilize the distance between a target C-H bond and a coordinating functional group, along with the geometry of the cyclic transition state in directed C-H activation, as core molecular recognition parameters to differentiate between multiple C-H bonds. In this Perspective, we discuss the advent and recent advances of this concept. We cover a wide range of transition-metal-catalyzed, template-directed remote C-H activation reactions of alcohols, carboxylic acids, sulfonates, phosphonates, and amines. Additionally, we review eminent examples which take advantage of non-covalent interactions to achieve regiocontrol. Continued advancement of this distance- and geometry-based differentiation approach for regioselective remote C-H functionalization reactions may lead to the ultimate realization of molecular editing: the freedom to modify organic molecules at any site, in any order.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




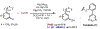




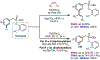

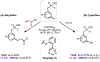
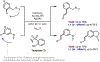



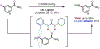
References
-
- Boger DL Modern Organic Synthesis: Lecture Notes. The Scripps Research Institute Press: La Jolla, 1999.
-
- Katsuki T; Sharpless KB The first practical method for asymmetric epoxidation. J. Am. Chem. Soc 1980, 102, 6974–5976.
- Hill JG; Sharpless KB; Exon CM; Regenye R Enantioselective epoxidation of allylic alcohols: (2s, 3s)-3-propyloxiranemethanol. Org. Synth 1985, 63, 66.
- Gao Y; Hanson RM; Klunder JM; Ko SY; Masamune H; Sharpless KB Catalytic asymmetric epoxidation and kinetic resolution: Modified procedures including in situ derivatization. J. Am. Chem. Soc 1987, 109, 5765–5780.
- Finn MG; Sharpless KB Mechanism of asymmetric epoxidation. 2. Catalyst structure. J. Am. Chem. Soc 1986, 2, 89–116.
-
- Whitehouse CJC; Bell SG; Wong L–L P450BM3 (CYP102A1): connecting the dots. Chem. Soc. Rev 2012, 41, 1218–1260. - PubMed
-
- Lichtor PA; Miller SJ Combinatorial evolution of site- and enantioselective catalysts for polyene epoxidation. Nat. Chem 2012, 4, 990–995. - PMC - PubMed
- Lichtor PA; Miller SJ Experimental lineage and functional analysis of a remotely directed peptide epoxidation catalyst. J. Am. Chem. Soc 2014, 136, 5301–5308. - PMC - PubMed
-
(c) Achieving site-selective catalysis is a broad issue surrounding many different types of functional groups. For a broader treatise, see:
- Kawabata T Site selective catalysis. Topics in Current Chemistry 2016, 372, Chapters 1–8. - PubMed
-
- Brown JM Directed homogeneous hydrogenation [New synthetic methods (65)]. Angew. Chem. Int. Ed 1987, 26, 190–203.
- Sehgal RK; Koenigsberger RU; Howard TJ Effect of ring size on hydrogenation of cyclic allylic alcohols. J. Org. Chem 1975, 40, 3073–3078.
- Halpern J; Harrod JF; James BR Homogeneous catalytic hydrogenation of olefinic compounds. J. Am. Chem. Soc 1961, 83, 753–754.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

