Efficacy and Safety of Integrated Traditional Chinese and Western Medicine for Corona Virus Disease 2019 (COVID-19): a systematic review and meta-analysis
- PMID: 32438037
- PMCID: PMC7211759
- DOI: 10.1016/j.phrs.2020.104896
Efficacy and Safety of Integrated Traditional Chinese and Western Medicine for Corona Virus Disease 2019 (COVID-19): a systematic review and meta-analysis
Abstract
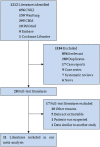
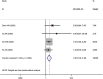
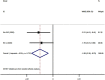
Corona virus disease (COVID-19) has now spread to all parts of the world and almost all countries are battling against it. This study aimed to assess the efficacy and safety of Integrated Traditional Chinese and Western Medicine (Hereinafter referred to as "Integrated Medicine") to COVID-19. We searched six major Chinese and English databases to identify randomized controlled trials (RCTs) and case-control studies (CCSs) of Integrated Medicine on COVID-19. Two reviewers independently screened, identified studies, and extracted data. Cochrane Risk of Bias tool and the Newcastle-Ottawa Scale were used to assess the quality of included RCTs and CCSs, respectively. Stata (version 13.0; StataCorp) was used to perform meta-analyses with the random-effects model. Risk ratio (RR) was used for dichotomous data while the weighted mean difference (WMD) was adopted for continuous variables as effect size, both of which were demonstrated in effect size and 95% confidence intervals (CI). A total of 11 studies were included. Four were RCTs and seven were CCSs. The sample size of including studies ranged from 42 to 200 (total 982). The traditional Chinese medicine included Chinese medicine compound drugs (QingFei TouXie FuZhengFang) and Chinese patent medicine (e.g. Shufeng Jiedu Capsule, Lianhua Qingwen granules). Compared with the control group, the overall response rate [RR = 1.230, 95%CI (1.113, 1.359), P = 0.000], cure rate [RR = 1.604, 95%CI (1.181, 2.177), P = 0.002], severity illness rate [RR = 0.350, 95%CI (0.154, 0.792), P = 0.012], and hospital stay [WMD = -1.991, 95%CI (-3.278, -0.703), P = 0.002] of the intervention group were better. In addition, Integrated Medicine can improve the disappearance rate of fever, cough, expectoration, fatigue, chest tightness and anorexia and reduce patients' fever, and fatigue time (P < 0.05). This review found that Integrated Medicine had better effects and did not increase adverse drug reactions for COVID-19. More high-quality RCTs are needed in the future.
Keywords: COVID-19; Efficacy; Integrated Traditional Chinese and Western Medicine; Meta-analysis; Safety.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Traditional and complementary medicine during COVID-19 pandemic.Phytother Res. 2020 Dec;34(12):3083-3084. doi: 10.1002/ptr.6828. Epub 2020 Aug 12. Phytother Res. 2020. PMID: 32785960 Free PMC article. No abstract available.
Similar articles
-
Efficacy and safety of Lianhua Qingwen combined with conventional antiviral Western Medicine in the treatment of coronavirus disease (covid-19) in 2019: Protocol for a systematic review and meta-analysis.Medicine (Baltimore). 2020 Jul 24;99(30):e21404. doi: 10.1097/MD.0000000000021404. Medicine (Baltimore). 2020. PMID: 32791754 Free PMC article.
-
[Meta analysis on the treatment of coronavirus disease 2019 by traditional Chinese and Western medicine].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021 Jun;33(6):714-720. doi: 10.3760/cma.j.cn121430-20201215-00758. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021. PMID: 34296692 Chinese.
-
Traditional Chinese medicine Lianhua Qingwen treating corona virus disease 2019(COVID-19): Meta-analysis of randomized controlled trials.PLoS One. 2020 Sep 11;15(9):e0238828. doi: 10.1371/journal.pone.0238828. eCollection 2020. PLoS One. 2020. PMID: 32915877 Free PMC article.
-
Efficacy of Integrated Traditional Chinese and Western Medicine for Treating COVID-19: A Systematic Review and Meta-Analysis of RCTs.Front Public Health. 2021 Jul 8;9:622707. doi: 10.3389/fpubh.2021.622707. eCollection 2021. Front Public Health. 2021. PMID: 34307269 Free PMC article.
-
Traditional Chinese medicine to treat COVID-19: the importance of evidence-based research.Drug Discov Ther. 2020;14(3):149-150. doi: 10.5582/ddt.2020.03054. Drug Discov Ther. 2020. PMID: 32669523
Cited by
-
[Salvianolic acid B and its magnesium salt inhibit SARS-CoV-2 infection of Vero-E6 cells by blocking spike protein-mediated membrane fusion].Nan Fang Yi Ke Da Xue Xue Bao. 2021 Apr 20;41(4):475-482. doi: 10.12122/j.issn.1673-4254.2021.04.01. Nan Fang Yi Ke Da Xue Xue Bao. 2021. PMID: 33963705 Free PMC article. Chinese.
-
Chinese medical drugs for coronavirus disease 2019: A systematic review and meta-analysis.Integr Med Res. 2020 Sep;9(3):100477. doi: 10.1016/j.imr.2020.100477. Epub 2020 Jul 28. Integr Med Res. 2020. PMID: 32834992 Free PMC article. Review.
-
Traditional and complementary medicine during COVID-19 pandemic.Phytother Res. 2020 Dec;34(12):3083-3084. doi: 10.1002/ptr.6828. Epub 2020 Aug 12. Phytother Res. 2020. PMID: 32785960 Free PMC article. No abstract available.
-
Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms.Pharmacol Res. 2021 Jan;163:105224. doi: 10.1016/j.phrs.2020.105224. Epub 2020 Sep 29. Pharmacol Res. 2021. PMID: 33007416 Free PMC article. Review.
-
Maoto, a traditional herbal medicine, for post-exposure prophylaxis for Japanese healthcare workers exposed to COVID-19: A single center study.J Infect Chemother. 2022 Jul;28(7):907-911. doi: 10.1016/j.jiac.2022.03.014. Epub 2022 Mar 21. J Infect Chemother. 2022. PMID: 35361537 Free PMC article.
References
-
- W.H.O., Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. Jan 11, 2020. https://www.who.int/internal-publications-detail/clinical-management-of-... (accessed 24 March 2020).
-
- Coronavirus Outbreak, available at: https://www.worldometers.info/coronavirus/. (accessed 27 March 2020).
-
- General Office of the National Health and Health Commission, Office of the State Administration of Traditional Chinese Medicine. Notice on Issuing a New Coronary Virus Pneumonia Diagnosis and Treatment guidelines (Trial Version 7) [EB/OL], http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb19..., (accessed 4 March 2020).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

