Current Use of and Trends in Hematopoietic Cell Transplantation in the United States
- PMID: 32438042
- PMCID: PMC7404814
- DOI: 10.1016/j.bbmt.2020.04.013
Current Use of and Trends in Hematopoietic Cell Transplantation in the United States
Erratum in
-
Corrigendum to 'Current Use and Trends in Hematopoietic Cell Transplantation in the United States' [Transplantation and Cellular Therapy 26/8 (2020) e177-e182].Transplant Cell Ther. 2022 Oct;28(10):715-716. doi: 10.1016/j.jtct.2021.02.006. Epub 2021 Mar 8. Transplant Cell Ther. 2022. PMID: 36202526 No abstract available.
Abstract
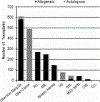
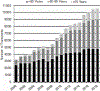
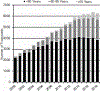
Hematopoietic cell transplantation (HCT) is a well-established treatment to control and/or cure many malignant and nonmalignant diseases involving the hematopoietic system and some solid tumors. We report information about HCT procedures performed in the United States in 2018 and analyze trends and outcomes of HCT as reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). Overall, compared with 2017, the number of allogeneic HCTs performed in the United States increased by 1%, and the number of autologous HCTs decreased by 5%. Key findings are fewer autologous HCTs performed for non-Hodgkin lymphoma and increasing numbers of haploidentical HCTs, nearly all of which use post-transplantation cyclophosphamide for graft-versus-host disease prophylaxis. There is a continuing increase in HCT in adults age >70 years, particularly for acute myelogenous leukemia and myelodysplastic syndromes. Survival rates by disease, disease stage, donor type, and age are presented. This report, prepared annually by the CIBMTR, provides a snapshot of current transplant activity in the United States.
Keywords: Activity; Hematopoietic cell transplantation; Summary slides.
Copyright © 2020 American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
Improving the Odds.Biol Blood Marrow Transplant. 2020 Aug;26(8):e173-e174. doi: 10.1016/j.bbmt.2020.04.017. Epub 2020 May 15. Biol Blood Marrow Transplant. 2020. PMID: 32417492 No abstract available.
References
-
- Kanate AS, Majhail NS, Savani BN, et al. : Indications for Hematopoietic Cell Transplantation and Immune Effector Cell Therapy: Guidelines from the American Society for Transplantation and Cellular Therapy: Guidelines for Hematopoietic Transplantation and Cellular Therapy. Biol Blood Marrow Transplant, 2020 - PubMed
-
- Jain T, Bar M, Kansagra AJ, et al. : Use of Chimeric Antigen Receptor T Cell Therapy in Clinical Practice for Relapsed/Refractory Aggressive B Cell Non-Hodgkin Lymphoma: An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant 25:2305–2321, 2019 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI126612/AI/NIAID NIH HHS/United States
- R21 HL140314/HL/NHLBI NIH HHS/United States
- K23 HL141445/HL/NHLBI NIH HHS/United States
- R01 CA231141/CA/NCI NIH HHS/United States
- U01 HL128568/HL/NHLBI NIH HHS/United States
- R01 HL129472/HL/NHLBI NIH HHS/United States
- P01 CA111412/CA/NCI NIH HHS/United States
- R01 CA218285/CA/NCI NIH HHS/United States
- R01 HL131731/HL/NHLBI NIH HHS/United States
- R01 HL126589/HL/NHLBI NIH HHS/United States
- R01 CA152108/CA/NCI NIH HHS/United States
- U24 CA076518/CA/NCI NIH HHS/United States
- U24 HL138660/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

