Hypermutation in single-stranded DNA
- PMID: 32438271
- PMCID: PMC7234795
- DOI: 10.1016/j.dnarep.2020.102868
Hypermutation in single-stranded DNA
Abstract
Regions of genomic DNA can become single-stranded in the course of normal replication and transcription as well as during DNA repair. Abnormal repair and replication intermediates can contain large stretches of persistent single-stranded DNA, which is extremely vulnerable to DNA damaging agents and hypermutation. Since such single-stranded DNA spans only a fraction of the genome at a given instance, hypermutation in these regions leads to tightly-spaced mutation clusters. This phenomenon of hypermutation in single-stranded DNA has been documented in several experimental models as well as in cancer genomes. Recently, hypermutated single-stranded RNA viral genomes also have been documented. Moreover, indications of hypermutation in single-stranded DNA may also be found in the human germline. This review will summarize key current knowledge and the recent developments in understanding the diverse mechanisms and sources of ssDNA hypermutation.
Keywords: Cancer; Hypermutation; Mutation clusters; Single-stranded DNA.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that there are no conflicts of interest.
Figures

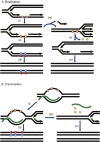
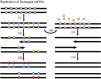
References
-
- Burch L.H., Yang Y., Sterling J.F., Roberts S.A., Chao F.G., Xu H., Zhang L., Walsh J., Resnick M.A., Mieczkowski P.A., Gordenin D.A. Damage-induced localized hypermutability. Cell Cycle. 2011;10:1073–1085. https://doi.org/15319 [pii] - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

