Thrombosis in patients with myeloma treated in the Myeloma IX and Myeloma XI phase 3 randomized controlled trials
- PMID: 32438407
- PMCID: PMC7453153
- DOI: 10.1182/blood.2020005125
Thrombosis in patients with myeloma treated in the Myeloma IX and Myeloma XI phase 3 randomized controlled trials
Erratum in
-
Bradbury CA, Craig Z, Cook G, et al. Thrombosis in patients with myeloma treated in the Myeloma IX and Myeloma XI phase 3 randomized controlled trials. Blood. 2020;136(9):1091-1104.Blood. 2020 Oct 22;136(17):1994. doi: 10.1182/blood.2020009029. Blood. 2020. PMID: 33091138 Free PMC article. No abstract available.
Abstract
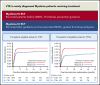
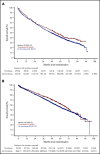
Newly diagnosed multiple myeloma (NDMM) patients treated with immunomodulatory drugs are at high risk of venous thromboembolism (VTE), but data are lacking from large prospective cohorts. We present thrombosis outcome data from Myeloma IX (n = 1936) and Myeloma XI (n = 4358) phase 3 randomized controlled trials for NDMM that treated transplant-eligible and transplant-ineligible patients before and after publication of thrombosis prevention guidelines. In Myeloma IX, transplant-eligible patients randomly assigned to cyclophosphamide, vincristine, doxorubicin, and dexamethasone (CVAD) induction had higher risk of VTE compared with patients treated with cyclophosphamide, thalidomide, and dexamethasone (CTD) (22.5% [n = 121 of 538] vs 16.1% [n = 89 of 554]; adjusted hazard ratio [aHR],1.46; 95% confidence interval [95% CI], 1.11-1.93). For transplant-ineligible patients, those randomly assigned to attenuated CTD (CTDa) induction had a higher risk of VTE compared with those treated with melphalan and prednisolone (MP) (16.0% [n = 68 of 425] vs 4.1% [n = 17 of 419]; aHR, 4.25; 95% CI, 2.50-7.20). In Myeloma XI, there was no difference in risk of VTE (12.2% [n = 124 of 1014] vs 13.2% [n = 133 of 1008]; aHR, 0.92; 95% CI, 0.72-1.18) or arterial thrombosis (1.2% [n = 12 of 1014] vs 1.5% [n = 15 of 1008]; aHR, 0.80; 95% CI, 0.37-1.70) between transplant-eligible pathways for patients treated with cyclophosphamide, lenalidomide, and dexamethasone (CRD) or CTD. For transplant-ineligible patients, there was no difference in VTEs between attenuated CRD (CRDa) and CTDa (10.4% [n = 95 of 916] vs 10.7% [n = 97 of 910]; aHR, 0.97; 95% CI, 0.73-1.29). However, arterial risk was higher with CRDa than with CTDa (3.1% [n = 28 of 916] vs 1.6% [n = 15 of 910]; aHR, 1.91; 95% CI, 1.02-3.57). Thrombotic events occurred almost entirely within 6 months of treatment initiation. Thrombosis was not associated with inferior progression-free survival (PFS) or overall survival (OS), apart from inferior OS for patients with arterial events (aHR, 1.53; 95% CI, 1.12-2.08) in Myeloma XI. The Myeloma XI trial protocol incorporated International Myeloma Working Group (IMWG) thrombosis prevention recommendations and compared with Myeloma IX, more patients received thromboprophylaxis (80.5% vs 22.3%) with lower rates of VTE for identical regimens (CTD, 13.2% vs 16.1%; CTDa, 10.7% vs 16.0%). However, thrombosis remained frequent in spite of IMWG-guided thromboprophylaxis, suggesting that new approaches are needed.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: C.A.B. received consultancy fees, honoraria, and speakers’ bureau fees from BMS, Pfizer, Novartis, Janssen, and Ablynx and funds to attend conferences from Bayer, Novartis, and Amgen. Z.C. received grants and nonfinancial support from Celgene, Merck Sharpe & Dohme, Amgen, and Takeda. G.C. received consultancy fees, honoraria, research funding, and speakers’ bureau fees from Takeda, Celgene, Janssen, and Amgen; consultancy fees and honoraria from Glycomimetics and Bristol-Myers Squibb; and consultancy fees, honoraria, and speakers’ bureau fees from Sanofi. C.P. received grants from Celgene. during the study and personal fees and nonfinancial support from Amgen, Takeda Oncology, Janssen, and Celgene outside the submitted work. D.A.C. received grants and nonfinancial support from Celgene, Merck Sharpe & Dohme, Amgen, and Takeda. A.H. received grants and nonfinancial support from Celgene, Merck Sharpe & Dohme, Amgen, and Takeda. M.W.J. received consultancy fees, honoraria, travel support, and research funding from Janssen; consultancy fees, honoraria, and travel support from Takeda and Amgen; consultancy fees, honoraria, and research funding from Celgene; and consultancy fees and honoraria from Novartis. J.R.J. received honoraria and research funding from Celgene. M.T.D. has equity ownership in and is on the board of directors and advisory committee for Abingdon Health. R.G.O. received honoraria and travel support from Takeda; consultancy fees and travel support from Janssen; and consultancy fees, honoraria, and research funding from Celgene Corporation. M.F.K. received consultancy fees and travel support from Bristol-Myers Squibb and Takeda; consultancy fees from Chugai; consultancy fees and honoraria from Janssen and Amgen; and consultancy fees, honoraria, and research funding from Celgene. W.M.G. received consultancy fees and research funding from Celgene; research funding from Amgen and Merck Sharp and Dohme; and honoraria from Janssen. F.E.D. received consultancy fees and honoraria from Amgen, AbbVie, Takeda, Janssen, and Celgene. G.J.M. received research funding from Janssen; consultancy fees and honoraria from Bristol-Myers Squibb and Takeda; and consultancy fees, honoraria, and research funding from Celgene. G.H.J. received consultancy fees, honoraria and speakers’ bureau fees from Roche, Amgen, Janssen, and Merck Sharp and Dohme; and consultancy fees, honoraria, travel support, research funding, and speakers’ bureau fees from Celgene and Takeda. The remaining authors declare no competing financial interests.
Figures


Comment in
-
Thrombosis in the modern era of multiple myeloma.Blood. 2020 Aug 27;136(9):1019-1021. doi: 10.1182/blood.2020006648. Blood. 2020. PMID: 32853375 No abstract available.
References
-
- Khorana A, Francis C, Culakova E, Kuderer N, Lyman G. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632-634. - PubMed
-
- Hunter R, Lewis S, Noble S, Rance J, Bennett P. “Post-thrombotic panic syndrome”: A thematic analysis of the experience of venous thromboembolism. Br J Health Psychol. 2017;22(1):8-25. - PubMed
-
- Barlogie B, Jagannath S, Desikan K, et al. . Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood. 1999;93(1):55-65. - PubMed
-
- Eby C. Pathogenesis and management of bleeding and thrombosis in plasma cell dyscrasias. Br J Haematol. 2009;145(2):151-163. - PubMed

