Redefining the multidimensional clinical phenotypes of frontotemporal lobar degeneration syndromes
- PMID: 32438414
- PMCID: PMC7241953
- DOI: 10.1093/brain/awaa097
Redefining the multidimensional clinical phenotypes of frontotemporal lobar degeneration syndromes
Abstract
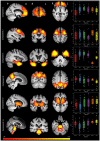
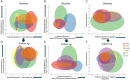
The syndromes caused by frontotemporal lobar degeneration have highly heterogeneous and overlapping clinical features. There has been great progress in the refinement of clinical diagnostic criteria in the past decade, but we propose that a better understanding of aetiology, pathophysiology and symptomatic treatments can arise from a transdiagnostic approach to clinical phenotype and brain morphometry. In a cross-sectional epidemiological study, we examined 310 patients with a syndrome likely to be caused by frontotemporal lobar degeneration, including behavioural variant frontotemporal dementia, non-fluent, and semantic variants of primary progressive aphasia (PPA), progressive supranuclear palsy and corticobasal syndrome. We included patients with logopenic PPA and those who met criteria for PPA but not a specific subtype. To date, 49 patients have a neuropathological diagnosis. A principal component analysis identified symptom dimensions that broadly recapitulated the core features of the main clinical syndromes. However, the subject-specific scores on these dimensions showed considerable overlap across the diagnostic groups. Sixty-two per cent of participants had phenotypic features that met the diagnostic criteria for more than one syndrome. Behavioural disturbance was prevalent in all groups. Forty-four per cent of patients with corticobasal syndrome had progressive supranuclear palsy-like features and 30% of patients with progressive supranuclear palsy had corticobasal syndrome-like features. Many patients with progressive supranuclear palsy and corticobasal syndrome had language impairments consistent with non-fluent variant PPA while patients with behavioural variant frontotemporal dementia often had semantic impairments. Using multivariate source-based morphometry on a subset of patients (n = 133), we identified patterns of covarying brain atrophy that were represented across the diagnostic groups. Canonical correlation analysis of clinical and imaging components found three key brain-behaviour relationships, with a continuous spectrum across the cohort rather than discrete diagnostic entities. In the 46 patients with follow-up (mean 3.6 years) syndromic overlap increased with time. Together, these results show that syndromes associated with frontotemporal lobar degeneration do not form discrete mutually exclusive categories from their clinical features or structural brain changes, but instead exist in a multidimensional spectrum. Patients often manifest diagnostic features of multiple disorders while deficits in behaviour, movement and language domains are not confined to specific diagnostic groups. It is important to recognize individual differences in clinical phenotype, both for clinical management and to understand pathogenic mechanisms. We suggest that a transdiagnostic approach to the spectrum of frontotemporal lobar degeneration syndromes provides a useful framework with which to understand disease aetiology, progression, and heterogeneity and to target future treatments to a higher proportion of patients.
Keywords: corticobasal syndrome; frontotemporal dementia; primary progressive aphasia; progressive supranuclear palsy; semantic dementia.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures





Comment in
-
Neither white nor black: embracing clinical variability in dementia diagnosis.Brain. 2020 May 1;143(5):1291-1293. doi: 10.1093/brain/awaa119. Brain. 2020. PMID: 32438412 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

