Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics
- PMID: 32438446
- PMCID: PMC7280633
- DOI: 10.1002/cpt.1909
Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics
Erratum in
-
CORRIGENDUM: Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics.Clin Pharmacol Ther. 2021 May;109(5):1362. doi: 10.1002/cpt.2116. Epub 2020 Dec 13. Clin Pharmacol Ther. 2021. PMID: 33870492 Free PMC article. No abstract available.
Abstract
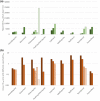
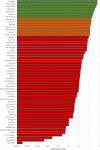
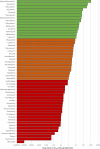
There is a rapidly expanding literature on the in vitro antiviral activity of drugs that may be repurposed for therapy or chemoprophylaxis against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2). However, this has not been accompanied by a comprehensive evaluation of the target plasma and lung concentrations of these drugs following approved dosing in humans. Accordingly, concentration 90% (EC90 ) values recalculated from in vitro anti-SARS-CoV-2 activity data was expressed as a ratio to the achievable maximum plasma concentration (Cmax ) at an approved dose in humans (Cmax /EC90 ratio). Only 14 of the 56 analyzed drugs achieved a Cmax /EC90 ratio above 1. A more in-depth assessment demonstrated that only nitazoxanide, nelfinavir, tipranavir (ritonavir-boosted), and sulfadoxine achieved plasma concentrations above their reported anti-SARS-CoV-2 activity across their entire approved dosing interval. An unbound lung to plasma tissue partition coefficient (Kp Ulung ) was also simulated to derive a lung Cmax /half-maximal effective concentration (EC50 ) as a better indicator of potential human efficacy. Hydroxychloroquine, chloroquine, mefloquine, atazanavir (ritonavir-boosted), tipranavir (ritonavir-boosted), ivermectin, azithromycin, and lopinavir (ritonavir-boosted) were all predicted to achieve lung concentrations over 10-fold higher than their reported EC50 . Nitazoxanide and sulfadoxine also exceeded their reported EC50 by 7.8-fold and 1.5-fold in lung, respectively. This analysis may be used to select potential candidates for further clinical testing, while deprioritizing compounds unlikely to attain target concentrations for antiviral activity. Future studies should focus on EC90 values and discuss findings in the context of achievable exposures in humans, especially within target compartments, such as the lungs, in order to maximize the potential for success of proposed human clinical trials.
© 2020 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
D.J.B. has received honoraria or advisory board payments from AbbVie, Gilead, ViiV, Merck, Janssen, and educational grants from AbbVie, Gilead, ViiV, Merck, Janssen, and Novartis. A.O. and S.P.R. are Directors of Tandem Nano Ltd. A.O. has received research funding from ViiV, Merck, Janssen, and consultancy from Gilead, ViiV and Merck not related to the current paper. P.O.N. is currently engaged in a collaboration with Romark LLC but this interaction did not influence the prioritization or conclusions in the current paper. All other authors declared no competing interests for this work.
Figures


References
-
- World Health Organisation . COVID‐19 Trials ‐ International Clinical Trials Registry Platform (ICTRP) <https://www.who.int/ictrp/search/en/> (2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

