Mitochondrial Function in Modulating Human Granulosa Cell Steroidogenesis and Female Fertility
- PMID: 32438750
- PMCID: PMC7279321
- DOI: 10.3390/ijms21103592
Mitochondrial Function in Modulating Human Granulosa Cell Steroidogenesis and Female Fertility
Abstract
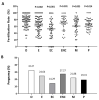
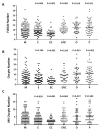
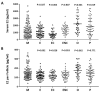
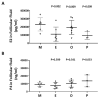
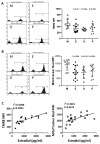
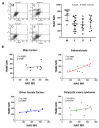
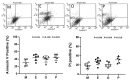
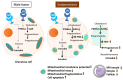
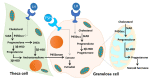
Ovarian follicle steroidogenesis associated with embryo quality results in a successful pregnancy. Each follicle consists of an oocyte surrounded by granulosa cells, which secrete several steroid and peptide hormones. Follicles harvested from women who conceived after assisted reproductive therapy (ART) had significantly higher estradiol levels in follicular fluids than the follicles from women who failed to conceive after ART. The higher follicular estradiol levels correlate well with successful fertilization following ART. Mitochondria are the central sites for steroid hormone biosynthesis. The first and rate-limiting step in the biosynthesis of steroid hormones occurs in the mitochondria of granulosa cells. In the present study, we hypothesized that the mitochondria in granulosa cells are critical for maintaining oocyte quality and fertility capacity. This study aims to clarify the relationship between mitochondrial function and granulosa cell steroidogenesis, and the relationship between hormone levels and fertility capacity. Sera, follicular fluids and granulosa cells were obtained from individuals undergoing IVF-ET treatment. The oocyte numbers, oocyte quality, fertilization rate, and pregnancy rate were also recorded. The patients who provided the granulosa cells were further classified into four groups: endometriosis, ovarian endometrioma, endometriosis without ovarian endometrioma, and polycystic ovary syndrome (PCOS); patients with other female factor infertility and male factor infertility were used as controls. We measured the levels of estradiol (E2) by radioimmunoassay. Concurrently, we analyzed the mitochondrial mass and membrane potential, and apoptosis by flow cytometry using nonyl acridine orange, TMRE, Annexin V-FITC and PI. Mitochondrial morphology was visualized by transfection with pLV-mitoDsRed. In addition, we assessed the protein levels of steroidogenic enzymes, steroidogenic acute regulatory protein (StAR) and 3β-hydroxysteroid dehydrogenase (3β-HSD) by Western blot. The results showed significantly decreased serum E2 and follicular E2 levels, and decreased IVF outcomes, in the patients with endometriosis. Reduced mitochondrial mass and decreased mitochondrial membrane potential were correlated with lower E2. Furthermore, a significant decrease in StAR and 3β-HSD was found in patients with ovarian endometrioma. The enzyme levels of StAR and 3β-HSD were highly correlated with E2 levels. Finally, elevated cumulus cell apoptosis was found in the patient group with ovarian endometrioma and PCOS. In conclusion, mitochondrial dysfunction of human granulosa cells may contribute to the decline of steroidogenesis, decreased fertilization rate, oocyte maturation rate, and oocyte quality, and it can ultimately jeopardize fertility.
Keywords: 3β-HSD.; StAR; estradiol; fertilization rate; granulosa cells; mitochondrial mass; progesterone; steroidogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Committee on Gynecologic Practice Committee opinion no. 618: Ovarian reserve testing. Obstet. Gynecol. 2015;125:268–273. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

