Oral Iron for IBD Patients: Lessons Learned at Time of COVID-19 Pandemic
- PMID: 32438763
- PMCID: PMC7290728
- DOI: 10.3390/jcm9051536
Oral Iron for IBD Patients: Lessons Learned at Time of COVID-19 Pandemic
Abstract
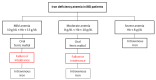
Anemia is a frequent manifestation in patients with chronic inflammatory bowel disease (IBD) and requires tight monitoring and adequate supplementary therapy. Intravenous iron is the first-line treatment in subjects with moderate-severe anemia, active disease, or oral iron intolerance. On the other hand, oral iron is recommended in patients with mild anemia and inactive disease. However, during the current coronavirus pandemic, hospital activities have significantly changed, and all non-essential procedures, including non-urgent iron infusions, have been rescheduled. Oral iron, including both the traditional formulations with ferrous iron and the new ferric iron complexes, could constitute a valid alternative for anemia treatment. For this reason, we conducted a literature review, to summarize the scientific evidence on oral iron therapy in IBD patients with anemia.
Keywords: COVID-19; anemia; inflammatory bowel disease; iron deficiency; oral iron.
Conflict of interest statement
F. D’Amico declares no conflict of interest. L. Peyrin-Biroulet personal fees from AbbVie, Janssen, Genentech, Ferring, Tillots, Pharmacosmos, Celltrion, Takeda, Boerhinger Ingelheim, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Alma, Sterna, Nestle, Enterome, Allergan, MSD, Roche, Arena, Gilead, Hikma, Amgen, BMS, Vifor, Norgine; Mylan, Lilly, Fresenius Kabi, Oppilan Pharma, Sublimity Therapeutics, Applied Molecular Transport, OSE Immunotherapeutics, Enthera, Theravance ; grants from Abbvie, MSD, Takeda ; stock-options CTMA. S Danese has served as a speaker, consultant, and advisory board member for Schering-Plough, AbbVie, Actelion, Alphawasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson and Johnson, Millenium Takeda, MSD, Nikkiso Europe GmbH, Novo Nordisk, Nycomed, Pfizer, Pharmacosmos, UCB Pharma, and Vifor.
Figures
References
-
- Harbord M., Annese V., Vavricka S.R., Allez M., Barreiro-de Acosta M., Boberg K.M., Burisch J., De Vos M., De Vries A.-M., Dick A.D., et al. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J. Crohns Colitis. 2016;10:239–254. doi: 10.1093/ecco-jcc/jjv213. - DOI - PMC - PubMed
-
- Bonovas S., Fiorino G., Allocca M., Lytras T., Tsantes A., Peyrin-Biroulet L., Danese S. Intravenous Versus Oral Iron for the Treatment of Anemia in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine. 2016;95:e2308. doi: 10.1097/MD.0000000000002308. - DOI - PMC - PubMed
-
- Dignass A.U., Gasche C., Bettenworth D., Birgegård G., Danese S., Gisbert J.P., Gomollon F., Iqbal T., Katsanos K., Koutroubakis I., et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J. Crohns Colitis. 2015;9:211–222. doi: 10.1093/ecco-jcc/jju009. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources