In Depth Analysis of the Contribution of Specific Glycoproteins to the Overall Bovine Whey N-Linked Glycoprofile
- PMID: 32438810
- PMCID: PMC7304067
- DOI: 10.1021/acs.jafc.0c00959
In Depth Analysis of the Contribution of Specific Glycoproteins to the Overall Bovine Whey N-Linked Glycoprofile
Abstract
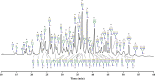
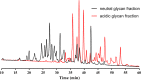
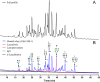
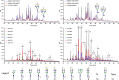
The N-linked glycoprofile of bovine whey is the combined result of individual protein glycoprofiles. In this work, we provide in-depth structural information on the glycan structures of known whey glycoproteins, namely, lactoferrin, lactoperoxidase, α-lactalbumin, immunoglobulin-G (IgG), and glycosylation-dependent cellular adhesion molecule 1 (GlyCAM-1, PP3). The majority (∼95%) of N-glycans present in the overall whey glycoprofile were attributed to three proteins: lactoferrin, IgG, and GlyCAM-1. We identified specific signature glycans for these main proteins; lactoferrin contributes oligomannose-type glycans, while IgG carries fucosylated di-antennary glycans with Gal-β(1,4)-GlcNAc (LacNAc) motifs. GlyCAM-1 is the sole whey glycoprotein carrying tri- and tetra-antennary structures, with a high degree of fucosylation and sialylation. Signature glycans can be used to recognize individual proteins in the overall whey glycoprofile as well as for protein concentration estimations. Application of the whey glycoprofile analysis to colostrum samples revealed dynamic protein concentration changes for IgG, lactoferrin, and GlyCAM-1 over time.
Keywords: GlyCAM-1; PP3; colostrum; milk; protein glycosylation; whey.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

