Optimized lactoferrin as a highly promising treatment for intracerebral hemorrhage: Pre-clinical experience
- PMID: 32438861
- PMCID: PMC7747168
- DOI: 10.1177/0271678X20925667
Optimized lactoferrin as a highly promising treatment for intracerebral hemorrhage: Pre-clinical experience
Abstract
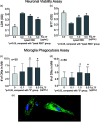
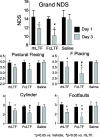
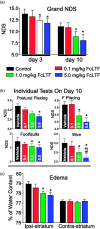
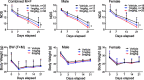
Intracerebral hemorrhage (ICH) is the deadliest form of stroke for which there is no effective treatment, despite an endless number of pre-clinical studies and clinical trials. The obvious therapeutic target is the neutralization of toxic products of red blood cell (RBC) lysis that lead to cytotoxicity, inflammation, and oxidative damage. We used rigorous approaches and translationally relevant experimental ICH models to show that lactoferrin-(LTF)-based monotherapy is uniquely robust in reducing brain damage after ICH. Specifically, we designed, produced, and pharmacokinetically/toxicologically characterized an optimized LTF, a fusion of human LTF and the Fc domain of human IgG (FcLTF) that has a 5.8-fold longer half-life in the circulation than native LTF. Following dose-optimization studies, we showed that FcLTF reduces neurological injury caused by ICH in aged male/female mice, and in young male Sprague Dawley (SD) and spontaneously hypertensive rats (SHR). FcLTF showed a remarkably long 24-h therapeutic window. In tissue culture systems, FcLTF protected neurons from the toxic effects of RBCs and promoted microglia toward phagocytosis of RBCs and dead neurons, documenting its pleotropic effect. Our findings indicate that FcLTF is safe and effective in reducing ICH-induced damage in animal models used in this study.
Keywords: Intracerebral hemorrhage; cytoprotection; lactoferrin; neuroprotection; phagocytosis.
Conflict of interest statement
Figures

Similar articles
-
Clearance of Neutrophils From ICH-Affected Brain by Macrophages Is Beneficial and Is Assisted by Lactoferrin and CD91.Stroke. 2024 Jan;55(1):166-176. doi: 10.1161/STROKEAHA.123.045194. Epub 2023 Dec 8. Stroke. 2024. PMID: 38063014 Free PMC article.
-
A cytotoxic effect of human lactoferrin fusion with Fc domain of IgG.Biometals. 2023 Jun;36(3):617-627. doi: 10.1007/s10534-022-00443-z. Epub 2022 Sep 22. Biometals. 2023. PMID: 36136256
-
Beneficial Role of Neutrophils Through Function of Lactoferrin After Intracerebral Hemorrhage.Stroke. 2018 May;49(5):1241-1247. doi: 10.1161/STROKEAHA.117.020544. Epub 2018 Apr 10. Stroke. 2018. PMID: 29636422 Free PMC article.
-
Lactoferrin and hematoma detoxification after intracerebral hemorrhage.Biochem Cell Biol. 2021 Feb;99(1):97-101. doi: 10.1139/bcb-2020-0116. Epub 2020 Sep 4. Biochem Cell Biol. 2021. PMID: 32886889 Review.
-
Therapeutic Potential of Drugs Targeting Pathophysiology of Intracerebral Hemorrhage: From Animal Models to Clinical Applications.Curr Pharm Des. 2017;23(15):2212-2225. doi: 10.2174/1381612822666161027151624. Curr Pharm Des. 2017. PMID: 27799045 Review.
Cited by
-
Microglial phagocytosis and regulatory mechanisms after stroke.J Cereb Blood Flow Metab. 2022 Sep;42(9):1579-1596. doi: 10.1177/0271678X221098841. Epub 2022 May 1. J Cereb Blood Flow Metab. 2022. PMID: 35491825 Free PMC article.
-
CNS-peripheral immune interactions in hemorrhagic stroke.J Cereb Blood Flow Metab. 2023 Feb;43(2):185-197. doi: 10.1177/0271678X221145089. Epub 2022 Dec 7. J Cereb Blood Flow Metab. 2023. PMID: 36476130 Free PMC article. Review.
-
Modulation of TDM-induced granuloma pathology by human lactoferrin: a persistent effect in mice.Biometals. 2023 Jun;36(3):603-615. doi: 10.1007/s10534-022-00434-0. Epub 2022 Aug 17. Biometals. 2023. PMID: 35976499
-
Neuroprotective Role of Lactoferrin during Early Brain Development and Injury through Lifespan.Nutrients. 2022 Jul 17;14(14):2923. doi: 10.3390/nu14142923. Nutrients. 2022. PMID: 35889882 Free PMC article. Review.
-
Clearance of Neutrophils From ICH-Affected Brain by Macrophages Is Beneficial and Is Assisted by Lactoferrin and CD91.Stroke. 2024 Jan;55(1):166-176. doi: 10.1161/STROKEAHA.123.045194. Epub 2023 Dec 8. Stroke. 2024. PMID: 38063014 Free PMC article.
References
-
- Urday S, Kimberly WT, Beslow LA, et al.. Targeting secondary injury in intracerebral haemorrhage–perihaematomal oedema. Nat Rev Neurol 2015; 11: 111–122. - PubMed
-
- Wagner KR, Sharp FR, Ardizzone TD, et al.. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab 2003; 23: 629–652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

