A randomized trial evaluating virus-specific effects of a combination probiotic in children with acute gastroenteritis
- PMID: 32439860
- PMCID: PMC7242434
- DOI: 10.1038/s41467-020-16308-3
A randomized trial evaluating virus-specific effects of a combination probiotic in children with acute gastroenteritis
Abstract
Gastroenteritis accounts for nearly 500,000 deaths in children younger than 5 years annually. Although probiotics have been touted as having the potential to expedite diarrhea resolution, recent clinical trials question their effectiveness. A potential explanation is a shift in pathogens following the introduction of a rotavirus vaccine. Here, we report the results of a multi-center, double-blind trial of 816 children with acute gastroenteritis who completed follow-up and provided multiple stool specimens. Participants were randomized to receive a probiotic containing Lactobacillus rhamnosus and Lactobacillus helveticus or placebo. We report no virus-specific beneficial effects attributable to the probiotic, either in reducing clinical symptoms or viral nucleic acid clearance from stool specimens collected up to 28 days following enrollment. We provide pathophysiological and microbiologic evidence to support the clinical findings and conclude that our data do not support routine probiotic administration to children with acute gastroenteritis, regardless of the infecting virus.
Conflict of interest statement
None of the authors have any potential or perceived conflicts of interest to declare. The study sponsors played no role in study design, conduct, data acquisition, analysis, manuscript preparation or the decision to submit the manuscript for publication. Dr. Stephen Freedman provides consulting services to Takeda Pharmaceuticals Inc. and was an invited speaker at the 10th Probiotics, Prebiotics and New Foods, Nutraceuticals and Botanicals for Nutrition and Human and Microbiota Health. PMS serves on advisory boards for Antibe Therapeutics, Cargill and Nestle-Gerber. He has received honoraria from Abbott Nutrition for speaking at continuing medical education activities and received research support from Lallemand Human Nutrition for a postdoctoral fellow award through a Mitacs Accelerates Internship.
Figures
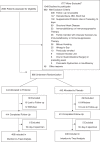
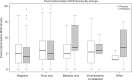


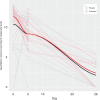
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

