Quality of Life in Japanese Patients with Dysmenorrhea Treated with Ethinylestradiol 20 μg/Drospirenone 3 mg in a Real-World Setting: An Observational Study
- PMID: 32440228
- PMCID: PMC7210450
- DOI: 10.2147/IJWH.S238460
Quality of Life in Japanese Patients with Dysmenorrhea Treated with Ethinylestradiol 20 μg/Drospirenone 3 mg in a Real-World Setting: An Observational Study
Abstract
Background: Dysmenorrhea affects approximately 80% of women in Japan and has a negative impact on health-related quality of life (HRQoL). Low-dose estrogen/progestin combined oral contraceptives have been shown to reduce the severity of dysmenorrhea symptoms. This study characterized HRQoL in Japanese women with dysmenorrhea before and after ethinylestradiol/drospirenone (EE/DRSP) treatment.
Methods: This prospective, observational study recruited 531 patients, of which 186 were evaluated after treatment with EE 20 μg/DRSP 3 mg for dysmenorrhea in a 24/4 cyclic regimen. The primary endpoints were mean baseline and post-treatment 36-Item Short-Form Health Survey version 2.0 (SF-36v2) scores for study patients compared with the general female population of Japan (calculated using norm-based scoring), and mean changes in study patient SF-36v2 scores between baseline and 6 to 8 treatment cycles.
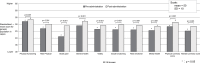
Results: Compared with Japanese norms, women with dysmenorrhea had lower pre-treatment SF-36v2 scores, except for the physical functioning domain. After 6-8 cycles of EE/DRSP treatment, all 8 SF-36v2 domain scores were significantly higher than baseline. The greatest improvements were observed in bodily pain and social functioning (mean change [standard deviation (SD)]: physical functioning: 1.4 [5.7], role physical: 3.2 [8.1], bodily pain: 7.8 [10.0], general health: 3.0 [7.0], vitality: 2.7 [8.1], social functioning: 3.5 [9.8], role emotional: 3.3 [9.2], and mental health: 3.0 [7.3]; p< 0.001 for all). Compared with the Japanese general population, study patients' post-treatment scores were significantly higher for the general health domain (p= 0.008) and physical summary scores (p= 0.033).
Conclusion: Dysmenorrhea has a profound impact on all aspects of functioning and well-being. This study, conducted in a real-world setting, found that physical, social, and mental HRQoL improved significantly after a cyclic regimen of EE/DRSP in Japanese patients with dysmenorrhea. This regimen may have the potential to provide an effective option to improve patient HRQoL.
Trial registration: Study sample was drawn from patients enrolled in a post-marketing surveillance study, registered June 20, 2011 (NCT01375998).
Keywords: Japan; SF-36; dysmenorrhea; ethinylestradiol/drospirenone; patient-reported outcomes; quality of life.
© 2020 Momoeda et al.
Conflict of interest statement
Mikio Momoeda was a paid medical advisor to Bayer Yakuhin, Ltd. for the duration of the study. Sayako Akiyama was an employee of Bayer Yakuhin, Ltd. at that time of the study. Kota Tanaka is an employee of EPS, which received funding from Bayer for the statistical analysis and reporting of study data. Yoshimi Suzukamo was a paid advisor on patient-reported outcome research to Bayer Yakuhin, Ltd. for the duration of the study. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
Quality of Life in Japanese Patients with Dysmenorrhea or Endometriosis-Associated Pelvic Pain Treated with Extended Regimen Ethinylestradiol/Drospirenone in a Real-World Setting: A Prospective Observational Study.Adv Ther. 2022 Nov;39(11):5087-5104. doi: 10.1007/s12325-022-02301-3. Epub 2022 Sep 2. Adv Ther. 2022. PMID: 36053449 Free PMC article.
-
Burden of Menstrual Pain Measured by Heatmap Visualization of Daily Patient-Reported Data in Japanese Patients Treated with Ethinylestradiol/Drospirenone: A Randomized Controlled Study.Int J Womens Health. 2020 Mar 10;12:175-185. doi: 10.2147/IJWH.S242864. eCollection 2020. Int J Womens Health. 2020. PMID: 32210639 Free PMC article.
-
Efficacy and safety of a flexible extended regimen of ethinylestradiol/drospirenone for the treatment of dysmenorrhea: a multicenter, randomized, open-label, active-controlled study.Int J Womens Health. 2017 May 2;9:295-305. doi: 10.2147/IJWH.S134576. eCollection 2017. Int J Womens Health. 2017. PMID: 28496369 Free PMC article.
-
Experiences with Yasmin: the acceptability of a novel oral contraceptive and its effect on well-being.Eur J Contracept Reprod Health Care. 2002 Dec;7 Suppl 3:35-41; discussion 42-3. Eur J Contracept Reprod Health Care. 2002. PMID: 12659405 Review.
-
Ethinylestradiol/drospirenone: a review of its use as an oral contraceptive.Treat Endocrinol. 2003;2(1):49-70. doi: 10.2165/00024677-200302010-00005. Treat Endocrinol. 2003. PMID: 15871554 Review.
Cited by
-
Trends in the estimated proportion of outpatients with menstrual disorders and the number of prescribed low-dose estrogen/progestin drugs in Japan: A descriptive study.PLoS One. 2025 Jul 18;20(7):e0327774. doi: 10.1371/journal.pone.0327774. eCollection 2025. PLoS One. 2025. PMID: 40680023 Free PMC article.
-
Effect and safety of drospirenone and ethinylestradiol tablets (II) for dysmenorrhea: A systematic review and meta-analysis.Front Med (Lausanne). 2022 Dec 15;9:938606. doi: 10.3389/fmed.2022.938606. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36590937 Free PMC article.
-
Quality of Life in Japanese Patients with Dysmenorrhea or Endometriosis-Associated Pelvic Pain Treated with Extended Regimen Ethinylestradiol/Drospirenone in a Real-World Setting: A Prospective Observational Study.Adv Ther. 2022 Nov;39(11):5087-5104. doi: 10.1007/s12325-022-02301-3. Epub 2022 Sep 2. Adv Ther. 2022. PMID: 36053449 Free PMC article.
-
Traditional Chinese medicine treatment strategies for primary dysmenorrhea.Front Endocrinol (Lausanne). 2025 May 2;16:1580051. doi: 10.3389/fendo.2025.1580051. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40385355 Free PMC article. Review.
References
-
- Momoeda M, Kondo M, Elliesen J, Yasuda M, Yamamoto S, Harada T. Efficacy and safety of a flexible extended regimen of ethinylestradiol/drospirenone for the treatment of dysmenorrhea: a multicenter, randomized, open-label, active-controlled study. Int J Women's Health. 2017;9:295–305. doi:10.2147/IJWH.S134576 - DOI - PMC - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical

