Toxicity testing is evolving!
- PMID: 32440338
- PMCID: PMC7233318
- DOI: 10.1093/toxres/tfaa011
Toxicity testing is evolving!
Abstract
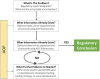
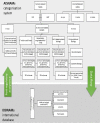
The efficient management of the continuously increasing number of chemical substances used in today's society is assuming greater importance than ever before. Toxicity testing plays a key role in the regulatory decisions of agencies and governments that aim to protect the public and the environment from the potentially harmful or adverse effects of these multitudinous chemicals. Therefore, there is a critical need for reliable toxicity-testing methods to identify, assess and interpret the hazardous properties of any substance. Traditionally, toxicity-testing approaches have been based on studies in experimental animals. However, in the last 20 years, there has been increasing concern regarding the sustainability of these methodologies. This has created a real need for the development of new approach methodologies (NAMs) that satisfy the regulatory requirements and are acceptable and affordable to society. Numerous initiatives have been launched worldwide in attempts to address this critical need. However, although the science to support this is now available, the legislation and the pace of NAMs acceptance is lagging behind. This review will consider some of the various initiatives in Europe to identify NAMs to replace or refine the current toxicity-testing methods for pharmaceuticals. This paper also presents a novel systematic approach to support the desired toxicity-testing methodologies that the 21st century deserves.
Keywords: 21st century toxicology; NAMs; Q(SAR); TOX21; TOX21c; animal testing; drug development; high-throughput screening; innovation; new approach methodologies; organ-on-a-chip; pharmaceuticals; read-across; regulatory toxicology; risk assessment; toxicity testing; toxicology.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Adeleye Y, Andersen M, Clewell R et al. . Implementing toxicity testing in the 21st century (TT21C): making safety decisions using toxicity pathways, and progress in a prototype risk assessment. Toxicology 2015;332:102–11. - PubMed
-
- Baird TJ, Caruso MJ, Gauvin DV et al. . NOEL and NOAEL: a retrospective analysis of mention in a sample of recently conducted safety pharmacology studies. J Pharmacol Toxicol Methods 2019;99:106597. - PubMed
-
- Horii I. The principle of safety evaluation in medicinal drug - how can toxicology contribute to drug discovery and development as a multidisciplinary science? J Toxicol Sci 2016;41:SP49–67PMID: 28250284. - PubMed
-
- LR DP. Alternative approaches in median lethality (LD50) and acute toxicity testing. Toxicol Lett 1989;49:159–70. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

