Halide Perovskites: Thermal Transport and Prospects for Thermoelectricity
- PMID: 32440477
- PMCID: PMC7237854
- DOI: 10.1002/advs.201903389
Halide Perovskites: Thermal Transport and Prospects for Thermoelectricity
Abstract
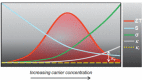
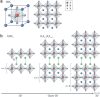
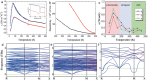
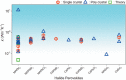
The recent re-emergence of halide perovskites has received escalating interest for optoelectronic applications. In addition to photovoltaics, the multifunctional nature of halide perovskites has led to diverse applications. The ultralow thermal conductivity coupled with decent mobility and charge carrier tunability led to the prediction of halide perovskites as a possible contender for future thermoelectrics. Herein, recent advances in thermal transport of halide perovskites and their potentials and challenges for thermoelectrics are reviewed. An overview of the phonon behavior in halide perovskites, as well as the compositional dependency is analyzed. Understanding thermal transport and knowing the thermal conductivity value is crucial for creating effective heat dissipation schemes and determining other thermal-related properties like thermo-optic coefficients, hot-carrier cooling, and thermoelectric efficiency. Recent works on halide perovskite-based thermoelectrics together with theoretical predictions for their future viability are highlighted. Also, progress on modulating halide perovskite-based thermoelectric properties using light and chemical doping is discussed. Finally, strategies to overcome the limiting factors in halide perovskite thermoelectrics and their prospects are emphasized.
Keywords: halide perovskites; hybrids; perovskites; phonons; thermal transport; thermoelectrics.
© 2020 The Authors. Published by WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Brenner T. M., Egger D. A., Kronik L., Hodes G., Cahen D., Nat. Rev. Mater. 2016, 1, 15007.
-
- Green M. A., Ho‐Baillie A., Snaith H. J., Nat. Photonics 2014, 8, 506.
-
- Park N.‐G., Grätzel M., Miyasaka T., Zhu K., Emery K., Nat. Energy 2016, 1, 16152.
-
- Stranks S. D., Eperon G. E., Grancini G., Menelaou C., Alcocer M. J., Leijtens T., Herz L. M., Petrozza A., Snaith H. J., Science 2013, 342, 341. - PubMed
-
- Xing G., Mathews N., Sun S., Lim S. S., Lam Y. M., Gratzel M., Mhaisalkar S., Sum T. C., Science 2013, 342, 344. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
