Inhibition of IRF5 cellular activity with cell-penetrating peptides that target homodimerization
- PMID: 32440537
- PMCID: PMC7228753
- DOI: 10.1126/sciadv.aay1057
Inhibition of IRF5 cellular activity with cell-penetrating peptides that target homodimerization
Abstract
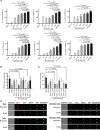
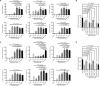
The transcription factor interferon regulatory factor 5 (IRF5) plays essential roles in pathogen-induced immunity downstream of Toll-, nucleotide-binding oligomerization domain-, and retinoic acid-inducible gene I-like receptors and is an autoimmune susceptibility gene. Normally, inactive in the cytoplasm, upon stimulation, IRF5 undergoes posttranslational modification(s), homodimerization, and nuclear translocation, where dimers mediate proinflammatory gene transcription. Here, we report the rational design of cell-penetrating peptides (CPPs) that disrupt IRF5 homodimerization. Biochemical and imaging analysis shows that IRF5-CPPs are cell permeable, noncytotoxic, and directly bind to endogenous IRF5. IRF5-CPPs were selective and afforded cell type- and species-specific inhibition. In plasmacytoid dendritic cells, inhibition of IRF5-mediated interferon-α production corresponded to a dose-dependent reduction in nuclear phosphorylated IRF5 [p(Ser462)IRF5], with no effect on pIRF5 levels. These data support that IRF5-CPPs function downstream of phosphorylation. Together, data support the utility of IRF5-CPPs as novel tools to probe IRF5 activation and function in disease.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




References
-
- Barnes B. J., Moore P. A., Pitha P. M., Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon α genes. J. Biol. Chem. 276, 23382–23390 (2001). - PubMed
-
- Barnes B. J., Richards M. J., Mancl M., Hanash S., Beretta L., Pitha P. M., Global and distinct targets of IRF-5 and IRF-7 during innate response to viral infection. J. Biol. Chem. 279, 45194–45207 (2004). - PubMed
-
- Barnes B. J., Kellum M. J., Pinder K. E., Frisancho J. A., Pitha P. M., IRF-5, a novel mediator of cell-cycle arrest and cell death. Cancer Res. 63, 6424–6431 (2003). - PubMed
-
- Takaoka A., Yanai H., Kondo S., Duncan G., Negishi H., Mizutani T., Kano S., Honda K., Ohba Y., Mak T. W., Taniguchi T., Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature 434, 243–249 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

