Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes
- PMID: 32440554
- PMCID: PMC7228761
- DOI: 10.1126/sciadv.aba4311
Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes
Erratum in
-
Erratum for the Research Article: "Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes".Sci Adv. 2023 Nov 24;9(47):eadl5234. doi: 10.1126/sciadv.adl5234. Epub 2023 Nov 24. Sci Adv. 2023. PMID: 38000036 Free PMC article. No abstract available.
Abstract
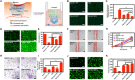
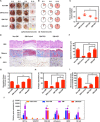
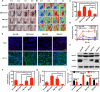
Chronic wounds in diabetes undergo a lifetime risk of developing into diabetic foot ulcers. Oxygen is crucial to wound healing by regulating cell proliferation, migration, and neovascularization. However, current oxygen therapies, including hyperbaric oxygen (HBO) and topical gaseous oxygen (TGO), mainly employ gaseous oxygen delivery, which is much less effective in penetrating the skin. Here, we introduce an oxygen-producing patch, made of living microalgae hydrogel, which can produce dissolved oxygen. The superior performance of the patch that results from its dissolved oxygen delivery is >100-fold much more efficient than TGO penetrating the skin. Further experiments indicate that the patch could promote cell proliferation, migration, and tube formation in vitro, and improve chronic wound healing and the survival of skin grafts in diabetic mice. We believe that the microalgae-gel patch can provide continuous dissolved oxygen to improve chronic wound healing.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

References
-
- International Diabetes Federation, IDF Diabetes Atlas (IDF, ed. 8, 2017).
-
- Hart T., Milner R., Cifu A., Management of a diabetic foot. JAMA 318, 1387–1388 (2017). - PubMed
-
- Thangarajah H., Vial I. N., Grogan R. H., Yao D., Shi Y., Januszyk M., Galiano R. D., Chang E. I., Galvez M. G., Glotzbach J. P., Wong V. W., Brownlee M., Gurtner G. C., HIF-1α dysfunction in diabetes. Cell Cycle 9, 75–79 (2010). - PubMed
-
- Thangarajah Y. D., Yao D., Chang E. I., Shi Y., Jazayeri L., Vial I. N., Galiano R. D., Du X. L., Grogan R., Galvez M. G., Januszyk M., Brownlee M., Gurtner G. C., The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc. Natl. Acad. Sci. U.S.A. 106, 13505–13510 (2009). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

