Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives
- PMID: 32441029
- PMCID: PMC7347247
- DOI: 10.1007/s12013-020-00913-6
Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives
Abstract
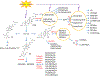
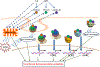
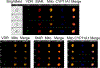
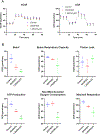
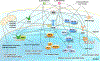
We have previously described new pathways of vitamin D3 activation by CYP11A1 to produce a variety of metabolites including 20(OH)D3 and 20,23(OH)2D3. These can be further hydroxylated by CYP27B1 to produce their C1α-hydroxyderivatives. CYP11A1 similarly initiates the metabolism of lumisterol (L3) through sequential hydroxylation of the side chain to produce 20(OH)L3, 22(OH)L3, 20,22(OH)2L3 and 24(OH)L3. CYP11A1 also acts on 7-dehydrocholesterol (7DHC) producing 22(OH)7DHC, 20,22(OH)27DHC and 7-dehydropregnenolone (7DHP) which can be converted to the D3 and L3 configurations following exposure to UVB. These CYP11A1-derived compounds are produced in vivo and are biologically active displaying anti-proliferative, anti-inflammatory, anti-cancer and pro-differentiation properties. Since the protective role of the classical form of vitamin D3 (1,25(OH)2D3) against UVB-induced damage is recognized, we recently tested whether novel CYP11A1-derived D3- and L3-hydroxyderivatives protect against UVB-induced damage in epidermal human keratinocytes and melanocytes. We found that along with 1,25(OH)2D3, CYP11A1-derived D3-hydroxyderivatives and L3 and its hydroxyderivatives exert photoprotective effects. These included induction of intracellular free radical scavenging and attenuation and repair of DNA damage. The protection of human keratinocytes against DNA damage included the activation of the NRF2-regulated antioxidant response, p53-phosphorylation and its translocation to the nucleus, and DNA repair induction. These data indicate that novel derivatives of vitamin D3 and lumisterol are promising photoprotective agents. However, detailed mechanisms of action, and the involvement of specific nuclear receptors, other vitamin D binding proteins or mitochondria, remain to be established.
Keywords: DNA damage; Lumisterol; Oxidative stress; Skin; Ultraviolet B; Vitamin D.
Conflict of interest statement
Conflict of Interest:
The authors declare that they have no conflict of interest.
Figures

References
-
- Holick MF & Clark MB The photobiogenesis and metabolism of vitamin D. Fed Proceed 37, 2567–2574 (1978). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

