Nanoparticle contrast agents for X-ray imaging applications
- PMID: 32441050
- PMCID: PMC7554158
- DOI: 10.1002/wnan.1642
Nanoparticle contrast agents for X-ray imaging applications
Abstract
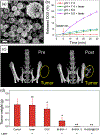
X-ray imaging is the most widely used diagnostic imaging method in modern medicine and several advanced forms of this technology have recently emerged. Iodinated molecules and barium sulfate suspensions are clinically approved X-ray contrast agents and are widely used. However, these existing contrast agents provide limited information, are suboptimal for new X-ray imaging techniques and are developing safety concerns. Thus, over the past 15 years, there has been a rapid growth in the development of nanoparticles as X-ray contrast agents. Nanoparticles have several desirable features such as high contrast payloads, the potential for long circulation times, and tunable physicochemical properties. Nanoparticles have also been used in a range of biomedical applications such as disease treatment, targeted imaging, and cell tracking. In this review, we discuss the principles behind X-ray contrast generation and introduce new types of X-ray imaging modalities, as well as potential elements and chemical compositions that are suitable for novel contrast agent development. We focus on the progress in nanoparticle X-ray contrast agents developed to be renally clearable, long circulating, theranostic, targeted, or for cell tracking. We feature agents that are used in conjunction with the newly developed multi-energy computed tomography and mammographic imaging technologies. Finally, we offer perspectives on current limitations and emerging research topics as well as expectations for the future development of the field. This article is categorized under: Diagnostic Tools > in vivo Nanodiagnostics and Imaging Nanotechnology Approaches to Biology > Nanoscale Systems in Biology.
Keywords: CT; X-ray imaging; cell tracking; dual-energy mammography; long circulating; nanoparticles; renal clearance; targeted agents; theranostics.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
Figures







References
-
- Alvarez RE, & Macovski A (1976). Energy-selective reconstructions in X-ray computerized tomography. Physics in Medicine and Biology, 21(5), 733–744. - PubMed
-
- Arifin DR, Long CM, Gilad AA, Alric C, Roux S, Tillement O, … Bulte JW (2011). Trimodal gadolinium-gold microcapsules containing pancreatic islet cells restore normoglycemia in diabetic mice and can be tracked by using US, CT, and positive-contrast MR imaging. Radiology, 260(3), 790–798. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

