Aberrant subchondral osteoblastic metabolism modifies NaV1.8 for osteoarthritis
- PMID: 32441256
- PMCID: PMC7308086
- DOI: 10.7554/eLife.57656
Aberrant subchondral osteoblastic metabolism modifies NaV1.8 for osteoarthritis
Abstract
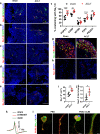
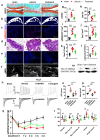
Pain is the most prominent symptom of osteoarthritis (OA) progression. However, the relationship between pain and OA progression remains largely unknown. Here we report osteoblast secret prostaglandin E2 (PGE2) during aberrant subchondral bone remodeling induces pain and OA progression in mice. Specific deletion of the major PGE2 producing enzyme cyclooxygenase 2 (COX2) in osteoblasts or PGE2 receptor EP4 in peripheral nerve markedly ameliorates OA symptoms. Mechanistically, PGE2 sensitizes dorsal root ganglia (DRG) neurons by modifying the voltage-gated sodium channel NaV1.8, evidenced by that genetically or pharmacologically inhibiting NaV1.8 in DRG neurons can substantially attenuate OA. Moreover, drugs targeting aberrant subchondral bone remodeling also attenuates OA through rebalancing PGE2 production and NaV1.8 modification. Thus, aberrant subchondral remodeling induced NaV1.8 neuronal modification is an important player in OA and is a potential therapeutic target in multiple skeletal degenerative diseases.
Keywords: Nav1.8; PGE2; human; human biology; medicine; mouse; neuroscience; osteoarthritis; osteoblast; pain.
Plain language summary
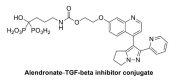
Many people will suffer from joint pain as they age, particularly in their knees. The most common cause of this pain is osteoarthritis, a disease that affects a tissue inside joints called cartilage. In a healthy knee, cartilage acts as a shock absorber. It cushions the ends of bones and enables them to move smoothly against one another. But in osteoarthritis, cartilage gradually wears away. As a result, the bones within a joint rub against each other whenever a person moves. This makes activities such as running or climbing stairs painful. But how does this pain arise? Previous work has implicated cells called osteoblasts. Osteoblasts are found in the area of the bone just below the cartilage. They produce new bone tissue throughout our lives, enabling our bones to regenerate and repair. Each time we move, forces acting on the knee joint activate osteoblasts. The cells respond by releasing a key molecule called PGE2, which is a factor in pain pathways. The joints of people with osteoarthritis produce too much PGE2. But exactly how this leads to increased pain sensation has been unclear. Zhu et al. now complete this story by working out how PGE2 triggers pain. Experiments in mice reveal that PGE2 irritates the nerve fibers that carry pain signals from the knee joint to the brain. It does this by activating a channel protein called Nav1.8, which allows sodium ions through the membranes of those nerve fibers. Zhu et al. show that, in a mouse model of osteoarthritis, Nav1.8 opens too widely in response to binding of PGE2, so the nerve cells become overactive and transmit a stronger pain sensation. This means that even small movements cause intense pain signals to travel from the joints to the brain. Building on their findings, Zhu et al. developed a drug that acts directly on bone to reduce PGE2 production, and show that this drug reduces pain in mice with osteoarthritis. At present, there are no treatments that reverse the damage that occurs during osteoarthritis, but further testing will determine whether this new drug could one day relieve joint pain in patients.
© 2020, Zhu et al.
Conflict of interest statement
JZ, GZ, SA, XW, MW, YL, ZC, YG, XD, YH, XC No competing interests declared
Figures










References
-
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nature Neuroscience. 1999;2:541–548. doi: 10.1038/9195. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

