Investigation of human trophoblast invasion in vitro
- PMID: 32441309
- PMCID: PMC7473396
- DOI: 10.1093/humupd/dmaa017
Investigation of human trophoblast invasion in vitro
Abstract
Background: In humans, inadequate trophoblast invasion into the decidua is associated with the 'great obstetrical syndromes' which include pre-eclampsia, foetal growth restriction (FGR) and stillbirth. The mechanisms regulating invasion remain poorly understood, although interactions with the uterine environment are clearly of central importance. Extravillous trophoblast (EVT) cells invade the uterus and transform the spiral arteries. Progress in understanding how they invade has been limited due to the lack of good in vitro models. Firstly, there are no non-malignant cell lines that have an EVT phenotype. Secondly, the invasion assays used are of limited use for the small numbers of primary EVT available from first-trimester placentas. We discuss recent progress in this field with the generation of new EVT lines and invasion assays using microfluidic technology.
Objective and rationale: Our aim is to describe the established models used to study human trophoblast invasion in vivo and in vitro. The difficulties of obtaining primary cells and cell lines that recapitulate the phenotype of EVT are discussed together with the advantages and pitfalls of the different invasion assays. We compare these traditional end point assays to microfluidic assays where the dynamics of migration can be measured.
Search methods: Relevant studies were identified by PubMed search, last updated on February 2020. A search was conducted to determine the number of journal articles published using the cell lines JEG-3, BeWo, JAR, HTR-8/Svneo, Swan-71 and primary human extravillous trophoblast in the last 5 years.
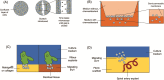
Outcomes: Deep trophoblast invasion into the maternal decidua is a particular feature of human pregnancy. This invasion needs to be finely regulated to allocate resources between mother and baby. A reliable source of EVT is needed to study in vitro how the uterine environment regulates this process. First, we critically discuss the issues with the trophoblast cell lines currently used; for example, most of them lack expression of the defining marker of EVT, HLA-G. Recently, advances in human stem cell and organoid technology have been applied to extraembryonic tissues to develop trophoblast cell lines that can grow in two (2D) and three dimensions (3D) and differentiate to EVT. This means that the 'trophoblast' cell lines currently in use should rapidly become obsolete. Second, we critically discuss the problems with assays to study trophoblast invasion. These lack physiological relevance and have simplified migration dynamics. Microfluidic assays are a powerful tool to study cell invasion because they require only a few cells, which are embedded in 3D in an extracellular matrix. Their major advantage is real-time monitoring of cell movement, enabling detailed analysis of the dynamics of trophoblast migration.
Wider implications: Trophoblast invasion in the first trimester of pregnancy remains poorly understood despite the importance of this process in the pathogenesis of pre-eclampsia, FGR, stillbirth and recurrent miscarriage. The new technologies described here will allow investigation into this critical process.
Keywords: human; invasion assays; microfluidics; organoids; trophoblast.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For permissions, please e-mail: journals.permission@oup.com.
Figures




References
-
- Abou-Kheir W, Barrak J, Hadadeh O. Daoud G. HTR-8/SVneo cell line contains a mixed population of cells. Placenta 2017;50:1–7. - PubMed
-
- Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol 2002;250:358–373. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

