Semaglutide 2.4 mg for the Treatment of Obesity: Key Elements of the STEP Trials 1 to 5
- PMID: 32441473
- PMCID: PMC7318657
- DOI: 10.1002/oby.22794
Semaglutide 2.4 mg for the Treatment of Obesity: Key Elements of the STEP Trials 1 to 5
Abstract
Objective: The obesity epidemic is a public health concern, warranting further research into pharmacological treatments for weight management (WM) as an adjunct to lifestyle interventions. The Semaglutide Treatment Effect in People with obesity (STEP) program aims to investigate the effect of semaglutide versus placebo on weight loss, safety, and tolerability in adults with obesity or overweight.
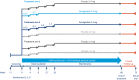
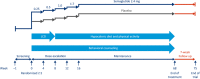
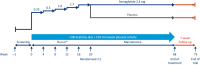
Methods: Across five phase 3 trials (NCT03548935, WM; NCT03552757, WM in type 2 diabetes; NCT03611582, WM with intensive behavioral therapy; NCT03548987, sustained WM; and NCT03693430, long-term WM), ~5,000 participants are being randomly assigned to receive semaglutide 2.4 mg once weekly subcutaneously versus placebo. Results will be available in 2020/2021. For all trials, the primary end point is change from baseline to end of treatment in body weight.
Results: Participants have a mean age of 46.2 to 55.3 years, are mostly female (mean: 74.1%-81.0%), and have a mean BMI of 35.7 to 38.5 kg/m2 and a mean waist circumference of 113.0 to 115.7 cm.
Conclusions: The STEP program evaluates the efficacy and safety of semaglutide 2.4 mg subcutaneously once weekly in a broad population. The trials will provide insights on WM in people with obesity with and without type 2 diabetes and on long-term follow-up.
© 2020 The Authors. Obesity published by Wiley Periodicals, Inc. on behalf of The Obesity Society (TOS).
Conflict of interest statement
RFK has served on advisory boards for Novo Nordisk, received grant support during the conduct of these trials, and received fees for participating in educational films from Novo Nordisk. SC is an employee of and holds stock in Novo Nordisk. MD has received grant support from Novo Nordisk, Sanofi, Eli Lilly, Boehringer Ingelheim, and Janssen; has served on advisory boards and received consulting and speaker fees from AstraZeneca and Janssen; and has served on advisory boards for Servier and received speaker fees from Mitsubishi Tanabe Pharma Corporation and Takeda. DD has received grant support from Novo Nordisk and Boehringer Ingelheim and has served on advisory boards for and received consulting and speaker fees from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, Teva, and Sanofi. WTG has served on advisory boards for Novo Nordisk, Sanofi, BOYDSense, Amgen, Gilead, and Boehringer Ingelheim and has received support for institutionally sponsored research from Sanofi, Pfizer, and Novo Nordisk. BG is an employee of and holds stock in Novo Nordisk. IL has received grant support from Novo Nordisk, Novartis, Pfizer, Merck, Mylan, and GI Dynamics; grant support for institutionally sponsored research from Novo Nordisk; and consulting fees from Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Sanofi, MannKind, Valeritas, Intarcia, and Janssen. MT is an employee of and holds stock in Novo Nordisk. TAW has served on advisory boards for Novo Nordisk and Weight Watchers and has received grant support, on behalf of the University of Pennsylvania, from Novo Nordisk. SW has received grant support and honoraria from and served on advisory boards for Novo Nordisk, Bausch Health, Eli Lilly, and Janssen and received nonfinancial support during the conduct of these trials. JPHW has received grant support from AstraZeneca, Novo Nordisk, and Takeda; has received personal fees from Boehringer Ingelheim, Napp, Novo Nordisk, Eli Lilly, Mundipharma, Sanofi, Janssen, and Takeda; and has served as a consultant for Astellas, AstraZeneca, Boehringer Ingelheim, Napp, Novo Nordisk, Eli Lilly, Mundipharma, Rhythm Pharmaceuticals, Sanofi, Janssen, and Wilmington Healthcare. DR has served on advisory boards for Arena, Eisai, Zafgen, and Novo Nordisk; has served as a clinical trials investigator for Bristol‐Myers Squibb, Merck, Nutrisource, Arena, Eisai, Vivus, Orexigen, Sanofi, AstraZenca, Novo Nordisk, and Weight Watchers; and has participated on a speaker’s bureau for Novo Nordisk and received grant support from Obeseinov SARL.
Figures


References
-
- Bray GA, Frühbeck G, Ryan DH, Wilding JPH. Management of obesity. Lancet 2016;387:1947‐1956. - PubMed
-
- Ralston J, Brinsden H, Buse K, et al. Time for a new obesity narrative. Lancet 2018;392:1384‐1386. - PubMed
-
- Ritten A, LaManna J. Unmet needs in obesity management: from guidelines to clinic. J Am Assoc Nurse Pract 2017;29:S30‐S42. - PubMed
-
- World Health Organization . Obesity and overweight. Updated February 16, 2018. Accessed October 25, 2019. https://www.who.int/news‐room/fact‐sheets/detail/obesity‐and‐overweight
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

