SIRT1-NOX4 signaling axis regulates cancer cachexia
- PMID: 32441762
- PMCID: PMC7336299
- DOI: 10.1084/jem.20190745
SIRT1-NOX4 signaling axis regulates cancer cachexia
Abstract
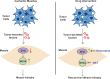
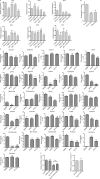
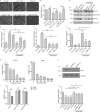
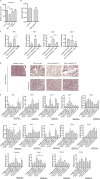
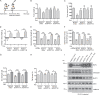
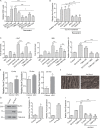
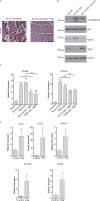
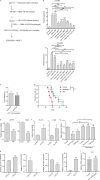
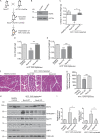
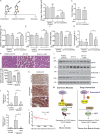
Approximately one third of cancer patients die due to complexities related to cachexia. However, the mechanisms of cachexia and the potential therapeutic interventions remain poorly studied. We observed a significant positive correlation between SIRT1 expression and muscle fiber cross-sectional area in pancreatic cancer patients. Rescuing Sirt1 expression by exogenous expression or pharmacological agents reverted cancer cell-induced myotube wasting in culture conditions and mouse models. RNA-seq and follow-up analyses showed cancer cell-mediated SIRT1 loss induced NF-κB signaling in cachectic muscles that enhanced the expression of FOXO transcription factors and NADPH oxidase 4 (Nox4), a key regulator of reactive oxygen species production. Additionally, we observed a negative correlation between NOX4 expression and skeletal muscle fiber cross-sectional area in pancreatic cancer patients. Knocking out Nox4 in skeletal muscles or pharmacological blockade of Nox4 activity abrogated tumor-induced cachexia in mice. Thus, we conclude that targeting the Sirt1-Nox4 axis in muscles is an effective therapeutic intervention for mitigating pancreatic cancer-induced cachexia.
© 2020 Dasgupta et al.
Conflict of interest statement
Disclosures: D.A. Tuveson reported "other" from Surface Oncology, Leap Therapeutics, and Cygnal Therapeutics; grants from ONO and Fibrogen; personal fees from Chugai and Merck outside the submitted work; SAB, stock from Surface Oncology, Leap Therapeutics, and Cygnal Therapeutics; sponsored research from ONO and Fibrogen; and honoraria from Chugai and Merck. No other disclosures were reported.
Figures



References
-
- Aapro M., Arends J., Bozzetti F., Fearon K., Grunberg S.M., Herrstedt J., Hopkinson J., Jacquelin-Ravel N., Jatoi A., Kaasa S., et al. ; ESMO (European School of Medical Oncology) . 2014. Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Ann. Oncol. 25:1492–1499. 10.1093/annonc/mdu085 - DOI - PubMed
-
- Abrego J., Gunda V., Vernucci E., Shukla S.K., King R.J., Dasgupta A., Goode G., Murthy D., Yu F., and Singh P.K.. 2017. GOT1-mediated anaplerotic glutamine metabolism regulates chronic acidosis stress in pancreatic cancer cells. Cancer Lett. 400:37–46. 10.1016/j.canlet.2017.04.029 - DOI - PMC - PubMed
-
- Amat R., Planavila A., Chen S.L., Iglesias R., Giralt M., and Villarroya F.. 2009. SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-gamma Co-activator-1alpha (PGC-1alpha) gene in skeletal muscle through the PGC-1alpha autoregulatory loop and interaction with MyoD. J. Biol. Chem. 284:21872–21880. 10.1074/jbc.M109.022749 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA216853/CA/NCI NIH HHS/United States
- R01 CA188134/CA/NCI NIH HHS/United States
- R01 HL091469/HL/NHLBI NIH HHS/United States
- R01 CA190092/CA/NCI NIH HHS/United States
- P30 CA036727/CA/NCI NIH HHS/United States
- R01 CA163649/CA/NCI NIH HHS/United States
- P30 CA045508/CA/NCI NIH HHS/United States
- T32 CA009476/CA/NCI NIH HHS/United States
- T32 CA148056/CA/NCI NIH HHS/United States
- P01 CA217798/CA/NCI NIH HHS/United States
- P50 CA127297/CA/NCI NIH HHS/United States
- R50 CA211462/CA/NCI NIH HHS/United States
- R01 CA210439/CA/NCI NIH HHS/United States
- U01 CA210240/CA/NCI NIH HHS/United States
- P30 GM103335/GM/NIGMS NIH HHS/United States
- R01 HL112330/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

