Antiviral treatment using the adenosine nucleoside analogue GS-441524 in cats with clinically diagnosed neurological feline infectious peritonitis
- PMID: 32441826
- PMCID: PMC7379040
- DOI: 10.1111/jvim.15780
Antiviral treatment using the adenosine nucleoside analogue GS-441524 in cats with clinically diagnosed neurological feline infectious peritonitis
Abstract
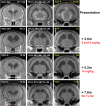
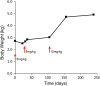
Feline infectious peritonitis (FIP) is caused by a mutant biotype of the feline enteric coronavirus. The resulting FIP virus (FIPV) commonly causes central nervous system (CNS) and ocular pathology in cases of noneffusive disease. Over 95% of cats with FIP will succumb to disease in days to months after diagnosis despite a variety of historically used treatments. Recently developed antiviral drugs have shown promise in treatment of nonneurological FIP, but data from neurological FIP cases are limited. Four cases of naturally occurring FIP with CNS involvement were treated with the antiviral nucleoside analogue GS-441524 (5-10 mg/kg) for at least 12 weeks. Cats were monitored serially with physical, neurologic, and ophthalmic examinations. One cat had serial magnetic resonance imaging (MRI), cerebrospinal fluid (CSF) analysis (including feline coronavirus [FCoV]) titers and FCoV reverse transcriptase [RT]-PCR) and serial ocular imaging using Fourier-domain optical coherence tomography (FD-OCT) and in vivo confocal microscopy (IVCM). All cats had a positive response to treatment. Three cats are alive off treatment (528, 516, and 354 days after treatment initiation) with normal physical and neurologic examinations. One cat was euthanized 216 days after treatment initiation following relapses after primary and secondary treatment. In 1 case, resolution of disease was defined based on normalization of MRI and CSF findings and resolution of cranial and caudal segment disease with ocular imaging. Treatment with GS-441524 shows clinical efficacy and may result in clearance and long-term resolution of neurological FIP. Dosages required for CNS disease may be higher than those used for nonneurological FIP.
Keywords: antiviral; cat; corona virus; ophthalmology.
© 2020 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals, Inc. on behalf of the American College of Veterinary Internal Medicine.
Conflict of interest statement
Authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

