Extracellular Vesicles Enhance the Remodeling of Cell-Free Silk Vascular Scaffolds in Rat Aortae
- PMID: 32441910
- PMCID: PMC12039313
- DOI: 10.1021/acsami.0c06609
Extracellular Vesicles Enhance the Remodeling of Cell-Free Silk Vascular Scaffolds in Rat Aortae
Abstract
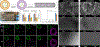
Vascular tissue engineering is aimed at developing regenerative vascular grafts to restore tissue function by bypassing or replacing defective arterial segments with tubular biodegradable scaffolds. Scaffolds are often combined with stem or progenitor cells to prevent acute thrombosis and initiate scaffold remodeling. However, there are limitations to cell-based technologies regarding safety and clinical translation. Extracellular vesicles (EVs) are nanosized particles released by most cell types, including stem and progenitor cells, that serve to transmit protein and RNA cargo to target cells throughout the body. EVs have been shown to replicate the therapeutic effect of their parent cells; therefore, EVs derived from stem or progenitor cells may serve as a more translatable, cell-free, therapeutic base for vascular scaffolds. Our study aims to determine if EV incorporation provides a positive effect on graft patency and remodeling in vivo. We first assessed the effect of human adipose-derived mesenchymal stem cell (hADMSC) EVs on vascular cells using in vitro bioassays. We then developed an EV-functionalized vascular graft by vacuum-seeding EVs into porous silk-based tubular scaffolds. These constructs were implanted as aortic interposition grafts in Lewis rats, and their remodeling capacity was compared to that observed for hADMSC-seeded and blank (non-seeded) controls. The EV group demonstrated improved patency (100%) compared to the hADMSC (56%) and blank controls (82%) following eight weeks in vivo. The EV group also produced significantly more elastin (126.46%) and collagen (44.59%) compared to the blank group, while the hADMSC group failed to produce significantly more elastin (57.64%) or collagen (11.21%) compared to the blank group. Qualitative staining of the explanted neo-tissue revealed improved endothelium formation, increased smooth muscle cell infiltration, and reduced macrophage numbers in the EV group compared to the controls, which aids in explaining this group's favorable pre-clinical outcomes.
Keywords: aortic graft; exosomes; mesenchymal stem cells; microvesicles; vascular tissue engineering.
Figures

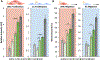


References
-
- Benjamin EJ; Blaha MJ; Chiuve SE; Cushman M; Das SR; Deo R; de Ferranti SD; Floyd J; Fornage M; Gillespie C;Isasi CR; Jimenez MC; Jordan LC; Judd SE; Lackland D; Lichtman JH; Lisabeth L; Liu S; Longenecker CT; Mackey RH; Matsushita K; Mozaffarian D; Mussolino ME; Nasir K; Neumar RW; Palaniappan L; Pandey DK; Thiagarajan RR; Reeves MJ; Ritchey M; Rodriguez CJ; Roth GA; Rosamond WD; Sasson C; Towfighi A; Tsao CW; Turner MB; Virani SS; Voeks JH; Willey JZ; Wilkins JT; Wu JH; Alger HM; Wong SS; Muntner P Heart Disease and Stroke Statistics–2017 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603. - PMC - PubMed
-
- Mendis S; Puska P; Norrving B Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organization: Geneva, Switzerland, 2011.
-
- Alexander JH; Smith PK Coronary-Artery Bypass Grafting. N. Engl. J. Med 2016, 374, 1954–1964. - PubMed
-
- Goodney PP; Beck AW; Nagle J; Welch HG; Zwolak RM National Trends in Lower Extremity Bypass Surgery, Endovascular Interventions, and Major Amputations. J. Vasc. Surg 2009, 50, 54–60. - PubMed
-
- Isenberg BC; Williams C; Tranquillo RT Small-Diameter Artificial Arteries Engineered in Vitro. Circ. Res 2006, 98, 25–35. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

