Cardiac mesenchymal cells from failing and nonfailing hearts limit ventricular dilation when administered late after infarction
- PMID: 32442025
- PMCID: PMC7474443
- DOI: 10.1152/ajpheart.00114.2020
Cardiac mesenchymal cells from failing and nonfailing hearts limit ventricular dilation when administered late after infarction
Abstract
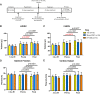
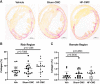
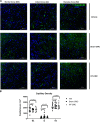
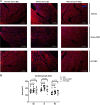
Although cell therapy-mediated cardiac repair offers promise for treatment/management of heart failure, lack of fundamental understanding of how cell therapy works limits its translational potential. In particular, whether reparative cells from failing hearts differ from cells derived from nonfailing hearts remains unexplored. Here, we assessed differences between cardiac mesenchymal cells (CMC) derived from failing (HF) versus nonfailing (Sham) hearts and whether the source of donor cells (i.e., from HF vs. Sham) limits reparative capacity, particularly when administered late after infarction. To determine the impact of the donor source of CMCs, we characterized the transcriptional profile of CMCs isolated from sham (Sham-CMC) and failing (HF-CMC) hearts. RNA-seq analysis revealed unique transcriptional signatures in Sham-CMC and HF-CMC, suggesting that the donor source impacts CMC. To determine whether the donor source affects reparative potential, C57BL6/J female mice were subjected to 60 min of regional myocardial ischemia and then reperfused for 35 days. In a randomized, controlled, and blinded fashion, vehicle, HF-CMC, or Sham-CMC were injected into the lumen of the left ventricle at 35 days post-MI. An additional 5 weeks later, cardiac function was assessed by echocardiography, which indicated that delayed administration of Sham-CMC and HF-CMC attenuated ventricular dilation. We also determined whether Sham-CMC and HF-CMC treatments affected ventricular histopathology. Our data indicate that the donor source (nonfailing vs. failing hearts) affects certain aspects of CMC, and these insights may have implications for future studies. Our data indicate that delayed administration of CMC limits ventricular dilation and that the source of CMC may influence their reparative actions.NEW & NOTEWORTHY Most preclinical studies have used only cells from healthy, nonfailing hearts. Whether donor condition (i.e., heart failure) impacts cells used for cell therapy is not known. We directly tested whether donor condition impacted the reparative effects of cardiac mesenchymal cells in a chronic model of myocardial infarction. Although cells from failing hearts differed in multiple aspects, they retained the potential to limit ventricular remodeling.
Keywords: cardiac repair; cell therapy; fibrosis; ventricular remodeling.
Conflict of interest statement
M.W. has pending intellectual property related to cell therapy. None of the other authors have any relevant conflicts to declare.
Figures


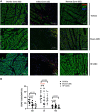
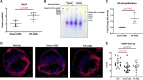
References
-
- Brainard RE, Watson LJ, Demartino AM, Brittian KR, Readnower RD, Boakye AA, Zhang D, Hoetker JD, Bhatnagar A, Baba SP, Jones SP. High fat feeding in mice is insufficient to induce cardiac dysfunction and does not exacerbate heart failure. PLoS One 8: e83174, 2013. [Erratum in: PLoS One 9: e113944, 2014.] 10.1371/journal.pone.0083174. - DOI - PMC - PubMed
-
- Cheng K, Malliaras K, Smith RR, Shen D, Sun B, Blusztajn A, Xie Y, Ibrahim A, Aminzadeh MA, Liu W, Li TS, De Robertis MA, Marbán L, Czer LS, Trento A, Marbán E. Human cardiosphere-derived cells from advanced heart failure patients exhibit augmented functional potency in myocardial repair. JACC Heart Fail 2: 49–61, 2014. doi:10.1016/j.jchf.2013.08.008. - DOI - PMC - PubMed
-
- Condorelli G, Roncarati R, Ross J Jr, Pisani A, Stassi G, Todaro M, Trocha S, Drusco A, Gu Y, Russo MA, Frati G, Jones SP, Lefer DJ, Napoli C, Croce CM. Heart-targeted overexpression of caspase3 in mice increases infarct size and depresses cardiac function. Proc Natl Acad Sci USA 98: 9977–9982, 2001. doi:10.1073/pnas.161120198. - DOI - PMC - PubMed
-
- Dassanayaka S, Brainard RE, Watson LJ, Long BW, Brittian KR, DeMartino AM, Aird AL, Gumpert AM, Audam TN, Kilfoil PJ, Muthusamy S, Hamid T, Prabhu SD, Jones SP. Cardiomyocyte Ogt limits ventricular dysfunction in mice following pressure overload without affecting hypertrophy. Basic Res Cardiol 112: 23, 2017. doi:10.1007/s00395-017-0612-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

