VTA Glutamatergic Neurons Mediate Innate Defensive Behaviors
- PMID: 32442399
- PMCID: PMC7381361
- DOI: 10.1016/j.neuron.2020.04.024
VTA Glutamatergic Neurons Mediate Innate Defensive Behaviors
Abstract
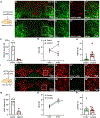
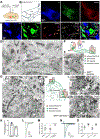
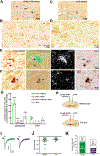
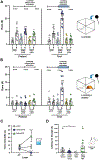
The ventral tegmental area (VTA) has dopamine, GABA, and glutamate neurons, which have been implicated in reward and aversion. Here, we determined whether VTA-glutamate or -GABA neurons play a role in innate defensive behavior. By VTA cell-type-specific genetic ablation, we found that ablation of glutamate, but not GABA, neurons abolishes escape behavior in response to threatening stimuli. We found that escape behavior is also decreased by chemogenetic inhibition of VTA-glutamate neurons and detected increases in activity in VTA-glutamate neurons in response to the threatening stimuli. By ultrastructural and electrophysiological analysis, we established that VTA-glutamate neurons receive a major monosynaptic glutamatergic input from the lateral hypothalamic area (LHA) and found that photoinhibition of this input decreases escape responses to threatening stimuli. These findings indicate that VTA-glutamate neurons are activated by and required for innate defensive responses and that information on threatening stimuli to VTA-glutamate neurons is relayed by LHA-glutamate neurons.
Keywords: VTA; VTA calcium imaging; VTA-GABA neurons; VTA-VGluT2 neurons; innate escape behavior; lateral hypothalamic area; looming; predator odor; threatening stimuli.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


References
-
- Berridge KC, and Robinson TE (1998) What is the role of dopamine in reward: Hedonic impacto, reward learning, or incentive salience? Brain Res. Brain Res. Rev 28, 309–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

