Nuclear-mitochondrial communication involving miR-181c plays an important role in cardiac dysfunction during obesity
- PMID: 32442661
- PMCID: PMC7483371
- DOI: 10.1016/j.yjmcc.2020.05.009
Nuclear-mitochondrial communication involving miR-181c plays an important role in cardiac dysfunction during obesity
Abstract
Aims: In cardiomyocytes, there is microRNA (miR) in the mitochondria that originates from the nuclear genome and matures in the cytoplasm before translocating into the mitochondria. Overexpression of one such miR, miR-181c, can lead to heart failure by stimulating reactive oxygen species (ROS) production and increasing mitochondrial calcium level ([Ca2+]m). Mitochondrial calcium uptake 1 protein (MICU1), a regulatory protein in the mitochondrial calcium uniporter complex, plays an important role in regulating [Ca2+]m. Obesity results in miR-181c overexpression and a decrease in MICU1. We hypothesize that lowering miR-181c would protect against obesity-induced cardiac dysfunction.
Methods and results: We used an in vivo mouse model of high-fat diet (HFD) for 18 weeks and induced high lipid load in H9c2 cells with oleate-conjugated bovine serum albumin in vitro. We tested the cardioprotective role of lowering miR-181c by using miR-181c/d-/- mice (in vivo) and AntagomiR against miR-181c (in vitro). HFD significantly upregulated heart levels of miR-181c and led to cardiac hypertrophy in wild-type mice, but not in miR-181c/d-/- mice. HFD also increased ROS production and pyruvate dehydrogenase activity (a surrogate for [Ca2+]m), but the increases were alleviated in miR-181c/d-/- mice. Moreover, miR-181c/d-/- mice fed a HFD had higher levels of MICU1 than did wild-type mice fed a HFD, attenuating the rise in [Ca2+]m. Overexpression of miR-181c in neonatal ventricular cardiomyocytes (NMVM) caused increased ROS production, which oxidized transcription factor Sp1 and led to a loss of Sp1, thereby slowing MICU1 transcription. Hence, miR-181c increases [Ca2+]m through Sp1 oxidation and downregulation of MICU1, suggesting that the cardioprotective effect of miR-181c/d-/- results from inhibition of Sp1 oxidation.
Conclusion: This study has identified a unique nuclear-mitochondrial communication mechanism in the heart orchestrated by miR-181c. Obesity-induced overexpression of miR-181c increases [Ca2+]m via downregulation of MICU1 and leads to cardiac injury. A strategy to inhibit miR-181c in cardiomyocytes can preserve cardiac function during obesity by improving mitochondrial function. Altering miR-181c expression may provide a pharmacologic approach to improve cardiomyopathy in individuals with obesity/type 2 diabetes.
Keywords: MICU1; Mitochondria; Mitochondrial calcium; Obesity; miR-181c; microRNA.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of Interest
None.
Figures
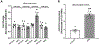



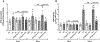


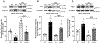
Similar articles
-
MICU3 Regulates Mitochondrial Calcium and Cardiac Hypertrophy.Circ Res. 2024 Jun 21;135(1):26-40. doi: 10.1161/CIRCRESAHA.123.324026. Epub 2024 May 15. Circ Res. 2024. PMID: 38747181 Free PMC article.
-
Sex-dependent phosphorylation of Argonaute 2 reduces the mitochondrial translocation of miR-181c and induces cardioprotection in females.J Mol Cell Cardiol. 2024 Sep;194:59-69. doi: 10.1016/j.yjmcc.2024.06.006. Epub 2024 Jun 14. J Mol Cell Cardiol. 2024. PMID: 38880194 Free PMC article.
-
miR-181c Activates Mitochondrial Calcium Uptake by Regulating MICU1 in the Heart.J Am Heart Assoc. 2019 Dec 17;8(24):e012919. doi: 10.1161/JAHA.119.012919. Epub 2019 Dec 5. J Am Heart Assoc. 2019. PMID: 31801413 Free PMC article.
-
Calcium Signaling and Reactive Oxygen Species in Mitochondria.Circ Res. 2018 May 11;122(10):1460-1478. doi: 10.1161/CIRCRESAHA.118.310082. Circ Res. 2018. PMID: 29748369 Review.
-
MICU1's calcium sensing beyond mitochondrial calcium uptake.Biochim Biophys Acta Mol Cell Res. 2024 Jun;1871(5):119714. doi: 10.1016/j.bbamcr.2024.119714. Epub 2024 Mar 29. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 38555977 Free PMC article. Review.
Cited by
-
The Role of the MiR-181 Family in Hepatocellular Carcinoma.Cells. 2024 Jul 31;13(15):1289. doi: 10.3390/cells13151289. Cells. 2024. PMID: 39120319 Free PMC article. Review.
-
Mitochondrial microRNAs (mitomiRs) as emerging biomarkers and therapeutic targets for chronic human diseases.Front Genet. 2025 Apr 25;16:1555563. doi: 10.3389/fgene.2025.1555563. eCollection 2025. Front Genet. 2025. PMID: 40352788 Free PMC article. Review.
-
MicroRNAs in cardiovascular diseases.Med Rev (2021). 2022 Apr 26;2(2):140-168. doi: 10.1515/mr-2021-0001. eCollection 2022 Apr. Med Rev (2021). 2022. PMID: 37724243 Free PMC article. Review.
-
MICU3 Regulates Mitochondrial Calcium and Cardiac Hypertrophy.Circ Res. 2024 Jun 21;135(1):26-40. doi: 10.1161/CIRCRESAHA.123.324026. Epub 2024 May 15. Circ Res. 2024. PMID: 38747181 Free PMC article.
-
Role of Specificity Protein 1 (SP1) in Cardiovascular Diseases: Pathological Mechanisms and Therapeutic Potentials.Biomolecules. 2024 Jul 7;14(7):807. doi: 10.3390/biom14070807. Biomolecules. 2024. PMID: 39062521 Free PMC article. Review.
References
-
- Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, Inflammation, and Cancer. Annu Rev Pathol. 2016;11:421–49. - PubMed
-
- Lovren F, Teoh H, Verma S. Obesity and atherosclerosis: mechanistic insights. Can J Cardiol. 2015;31(2):177–83. - PubMed
-
- Wang S, Wang C, Turdi S, Richmond KL, Zhang Y, Ren J. ALDH2 protects against high fat diet-induced obesity cardiomyopathy and defective autophagy: role of CaM kinase II, histone H3K9 methyltransferase SUV39H, Sirt1, and PGC-1alpha deacetylation. Int J Obes (Lond). 2018;42(5):1073–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

