Anlotinib combined with etoposide for platinum-resistant recurrent ovarian cancer: A case report
- PMID: 32443311
- PMCID: PMC7253621
- DOI: 10.1097/MD.0000000000020053
Anlotinib combined with etoposide for platinum-resistant recurrent ovarian cancer: A case report
Abstract
Introduction: Platinum-resistant ovarian cancer is characterized by its poor prognosis and limited treatment options. Angiogenesis plays a fundamental role in the development of drug-resistance in ovarian cancer. Anlotinib, a novel oral multi-targeted tyrosine kinase inhibitor which targets a board spectrum of angiogenesis-associated growth factor receptors, has shown promising anti-tumor efficacy in clinical trials. Herein, we report a case of ovarian cancer treated with anlotinib plus etoposide after secondary cytoreductive surgery.
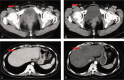
Patient concerns: A 45-year-old female with primary platinum-resistant ovarian cancer who progressed rapidly after the first cytoreductive surgery, the second cytoreductive surgery, and several lines of treatment. The patient refused to receive intravenous chemotherapy any more.
Diagnosis: Primary platinum-resistant ovarian cancer.
Interventions: The oral combination treatment of anlotinib (12 mg, qd) and etoposide (100 mg, qd) were delivered.
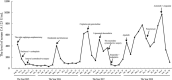
Outcomes: Finally, the patient was responsive to the orally treatment of anlotinib combined with etoposide. The patient has been alive with no evidence of disease progression for 18 weeks.
Conclusion: Our case suggests that oral treatment of anlotinib combined with etoposide which is acceptable and convenient, may be an additional option for the management of platinum-resistant ovarian cancer.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. - PubMed
-
- Cannistra SA. Cancer of the ovary. N Engl J Med 2004;351:2519–29. - PubMed
-
- Petrillo M, Pedone Anchora L, Tortorella L, et al. Secondary cytoreductive surgery in patients with isolated platinum-resistant recurrent ovarian cancer: a retrospective analysis. Gynecol Oncol 2014;134:257–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

