Upregulated and Hyperactivated Thalamic Connexin 43 Plays Important Roles in Pathomechanisms of Cognitive Impairment and Seizure of Autosomal Dominant Sleep-Related Hypermotor Epilepsy with S284L-Mutant α4 Subunit of Nicotinic ACh Receptor
- PMID: 32443400
- PMCID: PMC7280967
- DOI: 10.3390/ph13050099
Upregulated and Hyperactivated Thalamic Connexin 43 Plays Important Roles in Pathomechanisms of Cognitive Impairment and Seizure of Autosomal Dominant Sleep-Related Hypermotor Epilepsy with S284L-Mutant α4 Subunit of Nicotinic ACh Receptor
Abstract
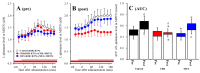
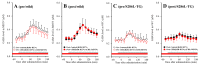
To understand the pathomechanism and pathophysiology of autosomal dominant sleep-related hypermotor epilepsy (ADSHE), we studied functional abnormalities of glutamatergic transmission in thalamocortical pathway from reticular thalamic nucleus (RTN), mediodorsal thalamic nucleus (MDTN) to orbitofrontal cortex (OFC) associated with S286L-mutant α4β2-nicotinic acetylcholine receptor (nAChR), and connexin43 (Cx43) hemichannel of transgenic rats bearing rat S286L-mutant Chrna4 gene (S286L-TG), corresponding to the human S284L-mutant CHRNA4 gene using simple Western analysis and multiprobe microdialysis. Cx43 expression in the thalamic plasma membrane fraction of S286L-TG was upregulated compared with that of wild-type. Subchronic administrations of therapeutic-relevant doses of zonisamide (ZNS) and carbamazepine (CBZ) decreased and did not affect Cx43 expression of S286L-TG, respectively. Upregulated Cx43 enhanced glutamatergic transmission during both resting and hyperexcitable stages in S286L-TG. Furthermore, activation of GABAergic transmission RTN-MDTN pathway conversely enhanced, but not inhibited, l-glutamate release in the MDTN via upregulated/activated Cx43. Local administration of therapeutic-relevant concentration of ZNS and CBZ acutely supressed and did not affect glutamatergic transmission in the thalamocortical pathway, respectively. These results suggest that pathomechanisms of ADSHE seizure and its cognitive deficit comorbidity, as well as pathophysiology of CBZ-resistant/ZNS-sensitive ADSHE seizures of patients with S284L-mutation.
Keywords: carbamazepine; cognition; connexin; hemichannel; idiopathic epilepsy; zonisamide.
Conflict of interest statement
The authors state no conflict of interest.
Figures








References
-
- Okada M., Zhu G., Yoshida S., Kaneko S. Validation criteria for genetic animal models of epilepsy. Epilepsy Seizure. 2010;3:109–120. doi: 10.3805/eands.3.109. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

