Phenylalanine: A Promising Inducer of Fruit Resistance to Postharvest Pathogens
- PMID: 32443417
- PMCID: PMC7278716
- DOI: 10.3390/foods9050646
Phenylalanine: A Promising Inducer of Fruit Resistance to Postharvest Pathogens
Abstract
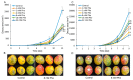
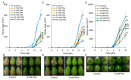
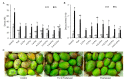
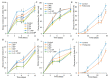
More than 40% of harvested fruit is lost, largely due to decay. In parallel, restrictions on postharvest fungicides call for eco-friendly alternatives. Fruit's natural resistance depends mainly on flavonoids and anthocyanins-which have antioxidant and antifungal activity-synthesized from the phenylpropanoid pathway with phenylalanine as a precursor. We hypothesized that phenylalanine could induce fruit's natural defense response and tolerance to fungal pathogens. The postharvest application of phenylalanine to mango and avocado fruit reduced anthracnose and stem-end rot caused by Colletotrichum gloeosporioides and Lasiodiplodia theobromae, respectively. The postharvest application of phenylalanine to citrus fruit reduced green mold caused by Penicillium digitatum. The optimal phenylalanine concentrations for postharvest application were 6 mM for citrus fruits and 8 mM for mangoes and avocadoes. The preharvest application of phenylalanine to strawberries, mangoes, and citrus fruits also reduced postharvest decay. Interestingly, citrus fruit resistance to P. digitatum inoculated immediately after phenylalanine application was not improved, whereas inoculation performed 2 days after phenylalanine treatment induced the defense response. Five hours after the treatment, no phenylalanine residue was detected on/in the fruit, probably due to rapid phenylalanine metabolism. Additionally, in vitro testing showed no inhibitory effect of phenylalanine on conidial germination. Altogether, we characterized a new inducer of the fruit defense response-phenylalanine. Preharvest or postharvest application to fruit led to the inhibition of fungal pathogen-induced postharvest decay, suggesting that the application of phenylalanine could become an eco-friendly and healthy alternative to fungicides.
Keywords: fungal pathogen; induced resistance; phenylalanine; postharvest application; postharvest decay; preharvest application.
Conflict of interest statement
The authors state no conflicts of interest.
Figures
References
-
- FAO Food Loss and Food Waste. [(accessed on 17 May 2020)]; Available online: http://www.fao.org/food-loss-and-food-waste/en/
-
- Wisniewski M., Droby S., Norelli J.L., Liu J., Schena L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Boil. Technol. 2016;122:3–10. doi: 10.1016/j.postharvbio.2016.05.012. - DOI
-
- Romanazzi G., Sanzani S.M., Bi Y., Tian S., Martínez P.G., Alkan N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Boil. Technol. 2016;122:82–94. doi: 10.1016/j.postharvbio.2016.08.003. - DOI
-
- Terry L.A., Joyce D.C. Elicitors of induced disease resistance in postharvest horticultural crops: A brief review. Postharvest Boil. Technol. 2004;32:1–13. doi: 10.1016/j.postharvbio.2003.09.016. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

