Administration of Defective Virus Inhibits Dengue Transmission into Mosquitoes
- PMID: 32443524
- PMCID: PMC7290595
- DOI: 10.3390/v12050558
Administration of Defective Virus Inhibits Dengue Transmission into Mosquitoes
Abstract
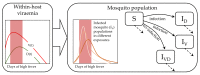
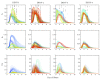
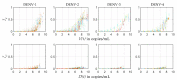
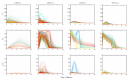
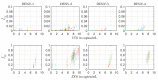
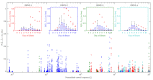
The host-vector shuttle and the bottleneck in dengue transmission is a significant aspect with regard to the study of dengue outbreaks. As mosquitoes require 100-1000 times more virus to become infected than human, the transmission of dengue virus from human to mosquito is a vulnerability that can be targeted to improve disease control. In order to capture the heterogeneity in the infectiousness of an infected patient population towards the mosquito population, we calibrate a population of host-to-vector virus transmission models based on an experimentally quantified infected fraction of a mosquito population. Once the population of models is well-calibrated, we deploy a population of controls that helps to inhibit the human-to-mosquito transmission of the dengue virus indirectly by reducing the viral load in the patient body fluid. We use an optimal bang-bang control on the administration of the defective virus (transmissible interfering particles (TIPs)) to symptomatic patients in the course of their febrile period and observe the dynamics in successful reduction of dengue spread into mosquitoes.
Keywords: defective particles; dengue transmission; dengue virus; mosquito infectious dose; population of models.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A population of bang-bang switches of defective interfering particles makes within-host dynamics of dengue virus controllable.PLoS Comput Biol. 2019 Nov 11;15(11):e1006668. doi: 10.1371/journal.pcbi.1006668. eCollection 2019 Nov. PLoS Comput Biol. 2019. PMID: 31710599 Free PMC article.
-
Field- and clinically derived estimates of Wolbachia-mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes.Proc Natl Acad Sci U S A. 2018 Jan 9;115(2):361-366. doi: 10.1073/pnas.1715788115. Epub 2017 Dec 26. Proc Natl Acad Sci U S A. 2018. PMID: 29279375 Free PMC article.
-
A predominant dengue virus-1 endemic strain and the vector competence of Aedes albopictus from Guangzhou City, China.Acta Trop. 2019 Nov;199:104975. doi: 10.1016/j.actatropica.2019.03.029. Epub 2019 Mar 31. Acta Trop. 2019. PMID: 30943381
-
The Global Expansion of Dengue: How Aedes aegypti Mosquitoes Enabled the First Pandemic Arbovirus.Annu Rev Entomol. 2020 Jan 7;65:191-208. doi: 10.1146/annurev-ento-011019-024918. Epub 2019 Oct 8. Annu Rev Entomol. 2020. PMID: 31594415 Review.
-
Aedes vittatus (Bigot) mosquito: An emerging threat to public health.J Vector Borne Dis. 2017 Oct-Dec;54(4):295-300. doi: 10.4103/0972-9062.225833. J Vector Borne Dis. 2017. PMID: 29460858 Review.
Cited by
-
Ingestion of amoxicillin-clavulanic acid at therapeutic concentration during blood meal impacts Aedes aegypti microbiota and dengue virus transmission.Sci Rep. 2024 Jun 13;14(1):13701. doi: 10.1038/s41598-024-64221-2. Sci Rep. 2024. PMID: 38871831 Free PMC article.
References
-
- Brady O.J., Gething P.W., Bhatt S., Messina J.P., Brownstein J.S., Hoen A.G., Moyes C.L., Farlow A.W., Scott T.W., Hay S.I. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

