The Zinc-Finger Domain Containing Protein ZC4H2 Interacts with TRPV4, Enhancing Channel Activity and Turnover at the Plasma Membrane
- PMID: 32443528
- PMCID: PMC7278933
- DOI: 10.3390/ijms21103556
The Zinc-Finger Domain Containing Protein ZC4H2 Interacts with TRPV4, Enhancing Channel Activity and Turnover at the Plasma Membrane
Abstract
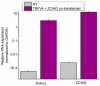
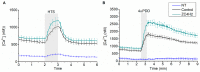
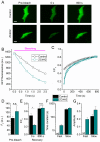
The Ca2+-permeable Transient Receptor Potential channel vanilloid subfamily member 4 (TRPV4) is involved in a broad range of physiological processes, including the regulation of systemic osmotic pressure, bone resorption, vascular tone, and bladder function. Mutations in the TRPV4 gene are the cause of a spectrum of inherited diseases (or TRPV4-pathies), which include skeletal dysplasias, arthropathies, and neuropathies. There is little understanding of the pathophysiological mechanisms underlying these variable disease phenotypes, but it has been hypothesized that disease-causing mutations affect interaction with regulatory proteins. Here, we performed a mammalian protein-protein interaction trap (MAPPIT) screen to identify proteins that interact with the cytosolic N terminus of human TRPV4, a region containing the majority of disease-causing mutations. We discovered the zinc-finger domain-containing protein ZC4H2 as a TRPV4-interacting protein. In heterologous expression experiments, we found that ZC4H2 increases both the basal activity of human TRPV4 as well as Ca2+ responses evoked by ligands or hypotonic cell swelling. Using total internal reflection fluorescence (TIRF) microscopy, we further showed that ZC4H2 accelerates TRPV4 turnover at the plasma membrane. Overall, these data demonstrate that ZC4H2 is a positive modulator of TRPV4, and suggest a link between TRPV4 and ZC4H2-associated rare disorders, which have several neuromuscular symptoms in common with TRPV4-pathies.
Keywords: Ca2+ signaling; TRPV4; ZC4H2; channel turnover; protein–protein interaction.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Auer-Grumbach M., Olschewski A., Papic L., Kremer H., McEntagart M.E., Uhrig S., Fischer C., Frohlich E., Balint Z., Tang B., et al. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat. Genet. 2010;42:160–164. doi: 10.1038/ng.508. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

