Experimental PCEP-Adjuvanted Swine Influenza H1N1 Vaccine Induced Strong Immune Responses but Did Not Protect Piglets against Heterologous H3N2 Virus Challenge
- PMID: 32443540
- PMCID: PMC7349969
- DOI: 10.3390/vaccines8020235
Experimental PCEP-Adjuvanted Swine Influenza H1N1 Vaccine Induced Strong Immune Responses but Did Not Protect Piglets against Heterologous H3N2 Virus Challenge
Abstract
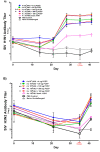
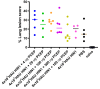
Vaccination is the most efficient method of protection against influenza infections. However, the rapidly mutating viruses and development of new strains make it necessary to develop new influenza vaccines annually. Hence, vaccines that stimulate cross-protection against multiple influenza subtypes are highly sought. Recent evidence suggests that adjuvants such as PCEP that promote Th1-type T cell and Th2-type T cell immune responses and broad-spectrum immune responses may confer cross-protection against heterologous influenza strains. In this study, we evaluated whether the immunogenic and protective potential of PCEP-adjuvanted inactivated swine influenza virus H1N1 vaccine can protect pigs immunized against live H3N2 virus. Piglets were vaccinated via the intradermal route with PCEP-adjuvanted inactivated swine influenza virus (SIV) H1N1 vaccine, boosted at day 21 with the same vaccines then challenged with infectious SIV H3N2 virus at day 35 via the tracheobronchial route. The pigs showed significant anti-H1N1 SIV specific antibody titres and H1N1 SIV neutralizing antibody titres, and these serum titres remained after the challenge with the H3N2 virus. In contrast, vaccination with anti-H1N1 SIV did not trigger anti-H3N2 SIV antibody titres or neutralizing antibody titres and these titres remained low until pigs were challenged with H3N2 SIV. At necropsy (six days after challenge), we collected prescapular lymph nodes and tracheobronchial draining the vaccination sites and challenge site, respectively. ELISPOTs from lymph node cells restimulated ex vivo with inactivated SIV H1N1 showed significant production of IFN-γ in the tracheobronchial cells, but not the prescapular lymph nodes. In contrast, lymph node cells restimulated ex vivo with inactivated SIV H1N1 showed significantly higher IL-13 and IL-17A in the prescapular lymph nodes draining the vaccination sites relative to unchallenged animals. Lung lesion scores show that intradermal vaccination with H1N1 SIV plus PCEP did not prevent lesions when the animals were challenged with H3N2. These results confirm previous findings that PCEP is effective as a vaccine adjuvant in that it induces strong immune responses and protects against homologous swine influenza H1N1 virus, but the experimental H1N1 vaccine failed to cross-protect against heterologous H3N2 virus.
Keywords: adjuvant; influenza; intradermal; pig; polyphosphazene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Vincent A.L., Ma W., Lager K.M., Janke B.H., Richt J.A. Swine influenza viruses: A North American perspective. Adv. Virus Res. 2008;72:127–154. - PubMed
-
- Brown I.H. History and Epidemiology of Swine Influenza in Europe. In: Richt J., Webby R., editors. Swine Influenza. Current Topics in Microbiology and Immunology. Volume 370 Springer; Berlin/Heidelberg, Germany: 2011. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

