Comparative Safety of Bevacizumab, Ranibizumab, and Aflibercept for Treatment of Neovascular Age-Related Macular Degeneration (AMD): A Systematic Review and Network Meta-Analysis of Direct Comparative Studies
- PMID: 32443612
- PMCID: PMC7291235
- DOI: 10.3390/jcm9051522
Comparative Safety of Bevacizumab, Ranibizumab, and Aflibercept for Treatment of Neovascular Age-Related Macular Degeneration (AMD): A Systematic Review and Network Meta-Analysis of Direct Comparative Studies
Abstract
Background: Since the efficacy of ranibizumab (RBZ), bevacizumab (BVZ) and aflibercept (AFB) is comparable in neovascular age-related macular degeneration (AMD), we conducted a systematic review and meta-analysis to evaluate the long-term safety profiles of these agents, including ocular safety.
Methods: Systematic review identifying randomized controlled trials (RCTs) comparing RBZ, BVZ and AFB directly published before March 2019. Serious ocular adverse events (SOAE) of special interest were endophthalmitis, pseudo-endophthalmitis, retinal pigment epithelium tear and newly identified macular atrophy.
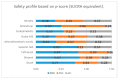
Results: Thirteen RCTs selected for meta-analysis (4952 patients, 8723 people-years follow-up): 10 compared RBZ vs. BVZ and three RBZ vs. AFB. There were no significant differences in almost all adverse events (systemic and ocular) between BVZ, RBZ and AFB in up to two years' follow-up. Macular atrophy was reported heterogeneously and not reported as SOAE in most trials.
Conclusions: Direct comparison of RBZ, BVZ and AFB safety profiles in the RCT network meta-analytical setting have not revealed a consistent benefit of these three commonly used anti-vascular endothelial growth factor (anti-VEGF) agents in AMD. Network model ranking highlighted potential benefits of RBZ in terms of a systemic safety profile; however, this appears a hypothesis rather than a conclusion. Newly identified macular atrophy is underestimated in RCTs-future real-world data should be focused on SOAE.
Keywords: aflibercept; anti-vascular endothelial growth factor; bevacizumab; meta-analysis; neovascular age-related macular degeneration; randomized controlled trials; ranibizumab.
Conflict of interest statement
Kirill Starostin is the Senior Medical Advisor in the Sanofi CHC Department. All other authors declare no conflicts of interest.
Figures


Similar articles
-
Real-World Effectiveness and Real-World Cost-Effectiveness of Intravitreal Aflibercept and Intravitreal Ranibizumab in Neovascular Age-Related Macular Degeneration: Systematic Review and Meta-Analysis of Real-World Studies.Adv Ther. 2020 Jan;37(1):300-315. doi: 10.1007/s12325-019-01147-6. Epub 2019 Nov 14. Adv Ther. 2020. PMID: 31728825 Free PMC article.
-
Anti-vascular endothelial growth factor treatment for retinal conditions: a systematic review and meta-analysis.BMJ Open. 2019 May 28;9(5):e022031. doi: 10.1136/bmjopen-2018-022031. BMJ Open. 2019. PMID: 31142516 Free PMC article.
-
Comparative effectiveness and harms of intravitreal antivascular endothelial growth factor agents for three retinal conditions: a systematic review and meta-analysis.Br J Ophthalmol. 2019 Apr;103(4):442-451. doi: 10.1136/bjophthalmol-2018-312691. Epub 2018 Nov 8. Br J Ophthalmol. 2019. PMID: 30409915
-
Effects of intraocular ranibizumab and bevacizumab in transgenic mice expressing human vascular endothelial growth factor.Ophthalmology. 2009 Sep;116(9):1748-54. doi: 10.1016/j.ophtha.2009.05.020. Epub 2009 Jul 29. Ophthalmology. 2009. PMID: 19643496 Free PMC article.
-
The role of Aflibercept in the management of age-related macular degeneration.Expert Opin Biol Ther. 2016;16(5):699-709. doi: 10.1517/14712598.2016.1167182. Expert Opin Biol Ther. 2016. PMID: 26982640 Review.
Cited by
-
Radiation retinopathy following episcleral brachytherapy for intraocular tumors: Current treatment options.J Contemp Brachytherapy. 2023 Oct;15(5):372-382. doi: 10.5114/jcb.2023.132398. Epub 2023 Oct 26. J Contemp Brachytherapy. 2023. PMID: 38026080 Free PMC article. Review.
-
Treatment of neovascular age-related macular degeneration with anti-vascular endothelial growth factor drugs: progress from mechanisms to clinical applications.Front Med (Lausanne). 2024 Jul 19;11:1411278. doi: 10.3389/fmed.2024.1411278. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39099595 Free PMC article. Review.
-
Bioengineering of Antibody Fragments: Challenges and Opportunities.Bioengineering (Basel). 2023 Jan 17;10(2):122. doi: 10.3390/bioengineering10020122. Bioengineering (Basel). 2023. PMID: 36829616 Free PMC article. Review.
-
[Frequency and distribution of the active agent of intravitreal injections in German centers 2015-2021-An oregis study].Ophthalmologie. 2024 Mar;121(3):196-206. doi: 10.1007/s00347-024-01986-x. Epub 2024 Feb 5. Ophthalmologie. 2024. PMID: 38315190 German.
-
A customized regimen of intravitreal aflibercept for the treatment of choroidal neovascularization secondary to different chorioretinal diseases.Int J Retina Vitreous. 2023 Jan 18;9(1):2. doi: 10.1186/s40942-023-00440-5. Int J Retina Vitreous. 2023. PMID: 36653854 Free PMC article.
References
-
- Chappelow A.V., Schachat A.P. Neovascular age-related macular degeneration. In: Nguyen Q., Rodrigues E., Farah M., Mieler W.F., editors. Retinal Pharmacotherapy. 1st, ed. Saunders; Philadelphia, PA, USA: 2010. pp. 128–132.
Publication types
LinkOut - more resources
Full Text Sources

