Fabrication of Transgelosomes for Enhancing the Ocular Delivery of Acetazolamide: Statistical Optimization, In Vitro Characterization, and In Vivo Study
- PMID: 32443679
- PMCID: PMC7284610
- DOI: 10.3390/pharmaceutics12050465
Fabrication of Transgelosomes for Enhancing the Ocular Delivery of Acetazolamide: Statistical Optimization, In Vitro Characterization, and In Vivo Study
Abstract
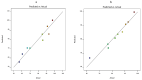
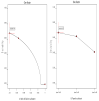
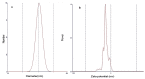
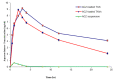
Acetazolamide (ACZ) is a potent carbonic anhydrase inhibitor that is used for the treatment of glaucoma. Its oral administration causes various undesirable side effects. This study aimed to formulate transgelosomes (TGS) for enhancing the ocular delivery of ACZ. ACZ-loaded transfersomes were formulated by the ethanol injection method, using phosphatidylcholine (PC) and different edge activators, including Tween 80, Span 60, and Cremophor RH 40. The effects of the ratio of lipid to surfactant and type of surfactant on % drug released after 8 h (Q8h) and entrapment efficiency (EE%) were investigated by using Design-Expert software. The optimized formula was formulated as TGS, using poloxamers as gelling agents. In vitro and in vivo characterization of ACZ-loaded TGS was performed. According to optimization study, F8 had the highest desirability value and was chosen as the optimized formula for preparing TGS. F8 appeared as spherical elastic nanovesicles with Q8h of 93.01 ± 3.76% and EE% of 84.44 ± 2.82. Compared to a free drug, TGS exhibited more prolonged drug release of 71.28 ± 0.46% after 8 h, higher ex vivo permeation of 66.82 ± 1.11% after 8 h and a significant lowering of intraocular pressure (IOP) for 24 h. Therefore, TGS provided a promising technique for improving the corneal delivery of ACZ.
Keywords: ACZ; corneal drug delivery; transfersomes; transgelosomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Nagasubramanian S., Bloom J., Poinoosawmy D., Hitchings R. Glaucoma Update III. Springer; Heidelberg, Germany: 1987. The effects of a topical acetazolamide preparation on intraocular pressure in patients with ocular hypertension; pp. 255–259.
LinkOut - more resources
Full Text Sources
Miscellaneous

