Present and Future of Bronchopulmonary Dysplasia
- PMID: 32443685
- PMCID: PMC7290764
- DOI: 10.3390/jcm9051539
Present and Future of Bronchopulmonary Dysplasia
Abstract
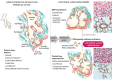
Bronchopulmonary dysplasia (BPD) is the most common respiratory disorder among infants born extremely preterm. The pathogenesis of BPD involves multiple prenatal and postnatal mechanisms affecting the development of a very immature lung. Their combined effects alter the lung's morphogenesis, disrupt capillary gas exchange in the alveoli, and lead to the pathological and clinical features of BPD. The disorder is ultimately the result of an aberrant repair response to antenatal and postnatal injuries to the developing lungs. Neonatology has made huge advances in dealing with conditions related to prematurity, but efforts to prevent and treat BPD have so far been only partially effective. Seeing that BPD appears to have a role in the early origin of chronic obstructive pulmonary disease, its prevention is pivotal also in long-term respiratory outcome of these patients. There is currently some evidence to support the use of antenatal glucocorticoids, surfactant therapy, protective noninvasive ventilation, targeted saturations, early caffeine treatment, vitamin A, and fluid restriction, but none of the existing strategies have had any significant impact in reducing the burden of BPD. New areas of research are raising novel therapeutic prospects, however. For instance, early topical (intratracheal or nebulized) steroids seem promising: they might help to limit BPD development without the side effects of systemic steroids. Evidence in favor of stem cell therapy has emerged from several preclinical trials, and from a couple of studies in humans. Mesenchymal stromal/stem cells (MSCs) have revealed a reparatory capability, preventing the progression of BPD in animal models. Administering MSC-conditioned media containing extracellular vesicles (EVs) have also demonstrated a preventive action, without the potential risks associated with unwanted engraftment or the adverse effects of administering cells. In this paper, we explore these emerging treatments and take a look at the revolutionary changes in BPD and neonatology on the horizon.
Keywords: bronchopulmonary dysplasia; extracellular vesicles; mesenchymal stem cells; mesenchymal stromal cells; neonatal ICU.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Stoll B.J., Hansen N.I., Bell E.F., Walsh M.C., Carlo W.A., Shankaran S., Laptook A.R., Sánchez P.J., Van Meurs K.P., Wyckoff M., et al. Trends in care practices, morbidity, and mortality of extremely preterm Neonates, 1993–2012. JAMA J. Am. Med. Assoc. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. - DOI - PMC - PubMed
-
- Devries L.B., Heyne R.J., Ramaciotti C., Brown L.S., Jaleel M.A., Kapadia V.S., Burchfield P.J., Brion L.P. Mortality among infants with evolving bronchopulmonary dysplasia increases with major surgery and with pulmonary hypertension. J. Perinatol. 2017;37:1043–1046. doi: 10.1038/jp.2017.89. - DOI - PubMed
-
- Lagatta J.M., Hysinger E.B., Zaniletti I., Wymore E.M., Vyas-Read S., Yallapragada S., Nelin L.D., Truog W.E., Padula M.A., Porta N.F.M., et al. The impact of pulmonary hypertension in preterm infants with severe bronchopulmonary dysplasia through 1 year. J. Pediatr. 2018 doi: 10.1016/j.jpeds.2018.07.035. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

