Lectins from the Edible Mushroom Agaricus bisporus and Their Therapeutic Potentials
- PMID: 32443732
- PMCID: PMC7287795
- DOI: 10.3390/molecules25102368
Lectins from the Edible Mushroom Agaricus bisporus and Their Therapeutic Potentials
Abstract
The mushroom Agaricus bisporus secretes biologically active compounds and proteins with benefits for human health. Most reported proteins from A. bisporus are tyrosinases and lectins. Lectins are of therapeutic or pharmaceutical interest. To date, only limited information is available on A. bisporus lectins and lectin-like proteins. No therapeutic products derived from A. bisporus lectin (ABL) are available on the market despite its extensive exploration. Recently, A. bisporus mannose-binding protein (Abmb) was discovered. Its discovery enriches the information and increases the interest in proteins with therapeutic potential from this mushroom. Furthermore, the A. bisporus genome reveals the possible occurrence of other lectins in this mushroom that may also have therapeutic potential. Most of these putative lectins belong to the same lectin groups as ABL and Abmb. Their relationship is discussed. Particular attention is addressed to ABL and Abmb, which have been explored for their potential in medicinal or pharmaceutical applications. ABL and Abmb have anti-proliferative activities toward cancer cells and a stimulatory effect on the immune system. Possible scenarios for their use in therapy and modification are also presented.
Keywords: anticancer; biological active fraction; immunomodulator; sugar-binding proteins; therapeutic protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



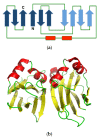
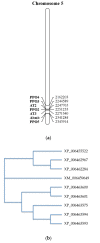
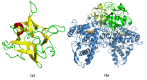

References
-
- Muszyńska B., Kała K., Rojowski J., Grzywacz A., Opoka W. Composition and biological properties of Agaricus bisporus fruiting bodies—A Review. Pol. J. Food Nutr. Sci. 2017;67:173–181. doi: 10.1515/pjfns-2016-0032. - DOI
-
- Eswari S., Saravana Bhavan P., Kalpana R., Dharani C., Manjula T., Sarumathi K., Rajkumar G. Phytochemical characterization of the mushroom Agaricus bisporus and assessment of its nutritional ability in the place of fishmeal for survival and growth of the freshwater prawn Macrobrachium rosenbergii post-larvae. Integr. Food Nutr. Metab. 2019;6:1–11.
-
- Dhamodharan G., Mirunalini S. A Novel Medicinal Characterization of Agaricus Bisporus (White Button Mushroom) Pharmacologyonline. 2010;2:456–463.
-
- Yu L.G., Fernig D.G., White M.R., Spiller D.G., Appleton P., Evans R.C., Grierson I., Smith J.A., Davies H., Gerasimenko O.V., et al. Edible mushroom (Agaricus bisporus) lectin, which reversibly inhibits epithelial cell proliferation, blocks nuclear localization sequence-dependent nuclear protein import. J. Biol. Chem. 1999;274:4890–4899. doi: 10.1074/jbc.274.8.4890. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

