A Convenient Synthesis of (16 S,20 S)-3β-hydroxy-5α-pregnane-20,16-carbolactam and its N-alkyl Derivatives
- PMID: 32443910
- PMCID: PMC7287600
- DOI: 10.3390/molecules25102377
A Convenient Synthesis of (16 S,20 S)-3β-hydroxy-5α-pregnane-20,16-carbolactam and its N-alkyl Derivatives
Abstract
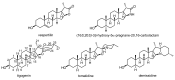
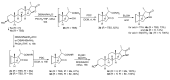
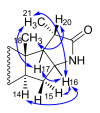
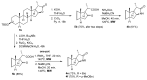
A concise synthesis of (16S,20S)-3β-hydroxy-5α-pregnane-20,16-carbolactam from tigogenin via the corresponding lactone is described. The most efficient synthetic route consisted of the lactone ring-opening with aminoalane reagent followed by PDC or Dess-Martin oxidation. The oxo-amide obtained was subjected to cyclization with Et3SiH/TFA or Et3SiH/Bi(TfO)3. Alternately, the lactone was converted first to the oxo-acid, which was then subjected to the microwave-assisted reductive amination. N-Alkyl derivatives were also obtained in a similar way.
Keywords: lactams; reductive amination; spirostane degradation; steroids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Synthesis and 5α-reductase inhibitory activity of C₂₁ steroids having 1,4-diene or 4,6-diene 20-ones and 4-azasteroid 20-oximes.Molecules. 2011 Dec 30;17(1):355-68. doi: 10.3390/molecules17010355. Molecules. 2011. PMID: 22210173 Free PMC article.
-
Conversion of tomato saponins to pregnane derivatives.Chem Pharm Bull (Tokyo). 2014;62(5):483-7. doi: 10.1248/cpb.c14-00004. Chem Pharm Bull (Tokyo). 2014. PMID: 24789931
-
Norrish-Prins reaction as a key step in the synthesis of 14β-hydroxy-5α (or 5β or Δ(5,6))-pregnane derivatives.Steroids. 2011 Sep-Oct;76(10-11):1166-75. doi: 10.1016/j.steroids.2011.05.004. Epub 2011 May 23. Steroids. 2011. PMID: 21645535
-
Conversion of spirostane and solanidane into pregnane (1)introduction of oxygen function into C-23.Chem Pharm Bull (Tokyo). 2012;60(1):150-3. doi: 10.1248/cpb.60.150. Chem Pharm Bull (Tokyo). 2012. PMID: 22223387
-
Steroids excreted in urine by neonates with 21-hydroxylase deficiency. 2. Characterization, using GC-MS and GC-MS/MS, of pregnanes and pregnenes with an oxo- group on the A- or B-ring.Steroids. 2012 Apr;77(5):382-93. doi: 10.1016/j.steroids.2011.12.018. Epub 2011 Dec 21. Steroids. 2012. PMID: 22210448
Cited by
-
Enhancing parathyroid preservation in papillary thyroid carcinoma surgery using nano-carbon suspension.Sci Rep. 2024 Oct 21;14(1):24680. doi: 10.1038/s41598-024-76126-1. Sci Rep. 2024. PMID: 39433967 Free PMC article.
References
-
- Gonzales A.G., Garcia F.C., Freire R., López E.S. Nuevas fuentes naturales de sapogeninas esteroidales. IX. Solanum Vespertilio Ait. An. Química. 1971;67:433–439.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

