Sensitive Aflatoxin B1 Detection Using Nanoparticle-Based Competitive Magnetic Immunodetection
- PMID: 32443933
- PMCID: PMC7290995
- DOI: 10.3390/toxins12050337
Sensitive Aflatoxin B1 Detection Using Nanoparticle-Based Competitive Magnetic Immunodetection
Abstract
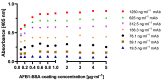
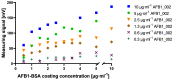
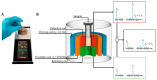
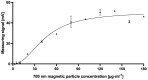
Food and crop contaminations with mycotoxins are a severe health risk for consumers and cause high economic losses worldwide. Currently, different chromatographic- and immuno-based methods are used to detect mycotoxins within different sample matrices. There is a need for novel, highly sensitive detection technologies that avoid time-consuming procedures and expensive laboratory equipment but still provide sufficient sensitivity to achieve the mandated detection limit for mycotoxin content. Here we describe a novel, highly sensitive, and portable aflatoxin B1 detection approach using competitive magnetic immunodetection (cMID). As a reference method, a competitive ELISA optimized by checkerboard titration was established. For the novel cMID procedure, immunofiltration columns, coated with aflatoxin B1-BSA conjugate were used for competitive enrichment of biotinylated aflatoxin B1-specific antibodies. Subsequently, magnetic particles functionalized with streptavidin can be applied to magnetically label retained antibodies. By means of frequency mixing technology, particles were detected and quantified corresponding to the aflatoxin content in the sample. After the optimization of assay conditions, we successfully demonstrated the new competitive magnetic detection approach with a comparable detection limit of 1.1 ng aflatoxin B1 per ml sample to the cELISA reference method. Our results indicate that the cMID is a promising method reducing the risks of processing contaminated commodities.
Keywords: frequency mixing technology; immunofiltration; magnetic beads; mycotoxin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Multifunctional magnetic bead-based electrochemical immunoassay for the detection of aflatoxin B1 in food.Analyst. 2009 Aug;134(8):1554-60. doi: 10.1039/b902401h. Epub 2009 May 20. Analyst. 2009. PMID: 20448920
-
A conventional chemical reaction for use in an unconventional assay: A colorimetric immunoassay for aflatoxin B1 by using enzyme-responsive just-in-time generation of a MnO2 based nanocatalyst.Mikrochim Acta. 2018 Jan 10;185(2):92. doi: 10.1007/s00604-017-2651-z. Mikrochim Acta. 2018. PMID: 29594447
-
Competitive horseradish peroxidase-linked aptamer assay for sensitive detection of Aflatoxin B1.Talanta. 2018 Mar 1;179:344-349. doi: 10.1016/j.talanta.2017.11.048. Epub 2017 Nov 21. Talanta. 2018. PMID: 29310242
-
Novel quartz crystal microbalance immunodetection of aflatoxin B1 coupling cargo-encapsulated liposome with indicator-triggered displacement assay.Anal Chim Acta. 2018 Nov 15;1031:161-168. doi: 10.1016/j.aca.2018.05.027. Epub 2018 May 9. Anal Chim Acta. 2018. PMID: 30119735
-
Recent advances in aflatoxin B1 detection based on nanotechnology and nanomaterials-A review.Anal Chim Acta. 2019 Sep 3;1069:1-27. doi: 10.1016/j.aca.2019.04.032. Epub 2019 Apr 19. Anal Chim Acta. 2019. PMID: 31084735 Review.
Cited by
-
Magnetic nanoparticles and magnetic particle spectroscopy-based bioassays: a 15 year recap.Nano Futures. 2022 Jun;6(2):022001. doi: 10.1088/2399-1984/ac5cd1. Epub 2022 Apr 7. Nano Futures. 2022. PMID: 36199556 Free PMC article.
-
Multiplex Detection of Magnetic Beads Using Offset Field Dependent Frequency Mixing Magnetic Detection.Sensors (Basel). 2021 Aug 31;21(17):5859. doi: 10.3390/s21175859. Sensors (Basel). 2021. PMID: 34502749 Free PMC article.
-
Development of Fast and Portable Frequency Magnetic Mixing-Based Serological SARS-CoV-2-Specific Antibody Detection Assay.Front Microbiol. 2021 May 5;12:643275. doi: 10.3389/fmicb.2021.643275. eCollection 2021. Front Microbiol. 2021. PMID: 34025604 Free PMC article.
-
Frequency Mixing Magnetic Detection Setup Employing Permanent Ring Magnets as a Static Offset Field Source.Sensors (Basel). 2022 Nov 14;22(22):8776. doi: 10.3390/s22228776. Sensors (Basel). 2022. PMID: 36433383 Free PMC article.
-
Automated Paper-Based Femtogram Sensing Device for Competitive Enzyme-Linked Immunosorbent Assay of Aflatoxin B1 Using Submicroliter Samples.Anal Chem. 2022 Mar 29;94(12):5099-5105. doi: 10.1021/acs.analchem.1c05401. Epub 2022 Mar 18. Anal Chem. 2022. PMID: 35302345 Free PMC article.
References
-
- Park D.L., Njapau H., Boutrif E. Minimizing Risks Posed by Mycotoxins Utilizing the HACCP Concept. Food, Nutrition and Agriculture; Rome, Italy: 1999. pp. 49–55.
-
- Blount W.P. Turkey “X” Disease. Turkeys. 1961;9:55–58, 61, 77.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

