Feasibility of direct venous inoculation of the radiation-attenuated Plasmodium falciparum whole sporozoite vaccine in children and infants in Siaya, western Kenya
- PMID: 32444192
- PMCID: PMC7297289
- DOI: 10.1016/j.vaccine.2020.05.008
Feasibility of direct venous inoculation of the radiation-attenuated Plasmodium falciparum whole sporozoite vaccine in children and infants in Siaya, western Kenya
Abstract
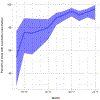
PfSPZ Vaccine, composed of radiation-attenuated, aseptic, purified, cryopreserved Plasmodium falciparum sporozoites, is administered by direct venous inoculation (DVI) for maximal efficacy against malaria. A critical issue for advancing vaccines that are administered intravenously is the ability to efficiently administer them across multiple age groups. As part of a pediatric safety, immunogenicity, and efficacy trial in western Kenya, we evaluated the feasibility and tolerability of DVI, including ease of venous access, injection time, and crying during the procedure across age groups. Part 1 was an age de-escalation, dose escalation trial in children aged 13 months-5 years and infants aged 5-12 months; part 2 was a vaccine efficacy trial including only infants, using the most skilled injectors from part 1. Injectors could use a vein viewer, if needed. A total of 1222 injections (target 0.5 mL) were initiated by DVI in 511 participants (36 were 5-9-year-olds, 65 were 13-59-month-olds, and 410 infants). The complete volume was injected in 1185/1222 (97.0%) vaccinations, 1083/1185 (91.4%) achieved with the first DVI. 474/511 (92.8%) participants received only complete injections, 27/511 (5.3%) received at least one partial injection (<0.5 mL), and in 10/511 (2.0%) venous access was not obtained. The rate of complete injections by single DVI for infants improved from 77.1% in part 1 to 92.8% in part 2. No crying occurred in 51/59 (86.4%) vaccinations in 5-9-year-olds, 25/86 (29.1%) vaccinations in 13-59-month-olds and 172/1067 (16.1%) vaccinations in infants. Mean administration time ranged from 2.6 to 4.6 minutes and was longer for younger age groups. These data show that vaccination by DVI was feasible and well tolerated in infants and children in this rural hospital in western Kenya, when performed by skilled injectors. We also report that shipping and storage in liquid nitrogen vapor phase was simple and efficient. (Clinicaltrials.gov NCT02687373).
Keywords: Africa; Direct venous inoculation; Feasibility; Infants; Malaria vaccine; Plasmodium falciparum whole sporozoite Vaccine.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [The following co-authors are or were employees of Sanaria (Rockville, MD), which manufactures the vaccine tested in this study: Abebe Y, Wijayalath W, James ER, Sim BKL, Billingsley PF, Richie TL, Hoffman SL].
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

