Experimental Antiglomerular Basement Membrane GN Induced by a Peptide from Actinomyces
- PMID: 32444356
- PMCID: PMC7269348
- DOI: 10.1681/ASN.2019060619
Experimental Antiglomerular Basement Membrane GN Induced by a Peptide from Actinomyces
Abstract
Background: Antiglomerular basement membrane (anti-GBM) disease is associated with HLA-DRB1*1501 (the major predisposing genetic factor in the disease), with α3127-148 as a nephritogenic T and B cell epitope. Although the cause of disease remains unclear, the association of infections with anti-GBM disease has been long suspected.
Methods: To investigate whether microbes might activate autoreactive T and B lymphocytes via molecular mimicry in anti-GBM disease, we used bioinformatic tools, including BLAST, SYFPEITHI, and ABCpred, for peptide searching and epitope prediction. We used sera from patients with anti-GBM disease to assess peptides recognized by antibodies, and immunized WKY rats and a humanized mouse model (HLA-DR15 transgenic mice) with each of the peptide candidates to assess pathogenicity.
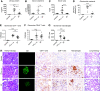
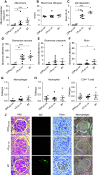
Results: On the basis of the critical motif, the bioinformatic approach identified 36 microbial peptides that mimic human α3127-148. Circulating antibodies in sera from patients with anti-GBM recognized nine of them. One peptide, B7, derived from Actinomyces species, induced proteinuria, linear IgG deposition on the GBM, and crescent formation when injected into WKY rats. The antibodies to B7 also targeted human and rat α3127-148. B7 induced T cell activation from human α3127-148-immunized rats. T cell responses to B7 were detected in rats immunized by Actinomyces lysate proteins or recombinant proteins. We confirmed B7's pathogenicity in HLA-DR15 transgenic mice that developed kidney injury similar to that observed in α3135-145-immunized mice.
Conclusions: Sera from patients with anti-GBM disease recognized microbial peptides identified through a bioinformatic approach, and a peptide from Actinomyces induced experimental anti-GBM GN by T and B cell crossreactivity. These studies demonstrate that anti-GBM disease may be initiated by immunization with a microbial peptide.
Keywords: glomerular disease; pathology; renal injury.
Copyright © 2020 by the American Society of Nephrology.
Figures




Comment in
-
Role of infection and molecular mimicry in the pathogenesis of anti-GBM disease.Nat Rev Nephrol. 2020 Aug;16(8):430. doi: 10.1038/s41581-020-0311-8. Nat Rev Nephrol. 2020. PMID: 32504073 No abstract available.
Similar articles
-
The pathogenicity of T cell epitopes on human Goodpasture antigen and its critical amino acid motif.J Cell Mol Med. 2017 Sep;21(9):2117-2128. doi: 10.1111/jcmm.13134. Epub 2017 Mar 10. J Cell Mol Med. 2017. PMID: 28296059 Free PMC article.
-
T cell epitope mimicry in antiglomerular basement membrane disease.J Immunol. 2006 Jan 15;176(2):1252-8. doi: 10.4049/jimmunol.176.2.1252. J Immunol. 2006. PMID: 16394016
-
HLA-DR15-specific inhibition attenuates autoreactivity to the Goodpasture antigen.J Autoimmun. 2019 Sep;103:102276. doi: 10.1016/j.jaut.2019.05.004. Epub 2019 May 16. J Autoimmun. 2019. PMID: 31104947
-
Anti-GBM glomerulonephritis: a T cell-mediated autoimmune disease?Arch Immunol Ther Exp (Warsz). 2004 Mar-Apr;52(2):96-103. Arch Immunol Ther Exp (Warsz). 2004. PMID: 15179323 Review.
-
Update on antiglomerular basement membrane disease.Curr Opin Rheumatol. 2011 Jan;23(1):32-7. doi: 10.1097/BOR.0b013e328341009f. Curr Opin Rheumatol. 2011. PMID: 21124085 Review.
Cited by
-
Lymphatic vessels in patients with crescentic glomerulonephritis: association with renal pathology and prognosis.J Nephrol. 2024 Jun;37(5):1285-1298. doi: 10.1007/s40620-024-01903-0. Epub 2024 Mar 25. J Nephrol. 2024. PMID: 38526665
-
The gut microbiome in microscopic polyangiitis with kidney involvement: common and unique alterations, clinical association and values for disease diagnosis and outcome prediction.Ann Transl Med. 2021 Aug;9(16):1286. doi: 10.21037/atm-21-1315. Ann Transl Med. 2021. PMID: 34532423 Free PMC article.
-
Perspectives in membranous nephropathy.Cell Tissue Res. 2021 Aug;385(2):405-422. doi: 10.1007/s00441-021-03429-4. Epub 2021 Apr 6. Cell Tissue Res. 2021. PMID: 33825066 Free PMC article. Review.
-
Efficacy and Safety of Rituximab in Antiglomerular Basement Membrane Disease.Kidney Int Rep. 2024 Dec 31;10(3):743-752. doi: 10.1016/j.ekir.2024.12.026. eCollection 2025 Mar. Kidney Int Rep. 2024. PMID: 40225363 Free PMC article.
-
Investigating the metabolite signature of an altered oral microbiota as a discriminant factor for multiple sclerosis: a pilot study.Sci Rep. 2024 Apr 2;14(1):7786. doi: 10.1038/s41598-024-57949-4. Sci Rep. 2024. PMID: 38565581 Free PMC article.
References
-
- Cui Z, Zhao MH: Advances in human antiglomerular basement membrane disease. Nat Rev Nephrol 7: 697–705, 2011. - PubMed
-
- Saus J, Wieslander J, Langeveld JP, Quinones S, Hudson BG: Identification of the Goodpasture antigen as the alpha 3(IV) chain of collagen IV. J Biol Chem 263: 13374–13380, 1988. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

