Antiviral Efficacies of FDA-Approved Drugs against SARS-CoV-2 Infection in Ferrets
- PMID: 32444382
- PMCID: PMC7244896
- DOI: 10.1128/mBio.01114-20
Antiviral Efficacies of FDA-Approved Drugs against SARS-CoV-2 Infection in Ferrets
Abstract
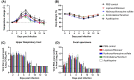
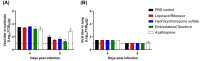
Due to the urgent need of a therapeutic treatment for coronavirus (CoV) disease 2019 (COVID-19) patients, a number of FDA-approved/repurposed drugs have been suggested as antiviral candidates at clinics, without sufficient information. Furthermore, there have been extensive debates over antiviral candidates for their effectiveness and safety against severe acute respiratory syndrome CoV 2 (SARS-CoV-2), suggesting that rapid preclinical animal studies are required to identify potential antiviral candidates for human trials. To this end, the antiviral efficacies of lopinavir-ritonavir, hydroxychloroquine sulfate, and emtricitabine-tenofovir for SARS-CoV-2 infection were assessed in the ferret infection model. While the lopinavir-ritonavir-, hydroxychloroquine sulfate-, or emtricitabine-tenofovir-treated group exhibited lower overall clinical scores than the phosphate-buffered saline (PBS)-treated control group, the virus titers in nasal washes, stool specimens, and respiratory tissues were similar between all three antiviral-candidate-treated groups and the PBS-treated control group. Only the emtricitabine-tenofovir-treated group showed lower virus titers in nasal washes at 8 days postinfection (dpi) than the PBS-treated control group. To further explore the effect of immune suppression on viral infection and clinical outcome, ferrets were treated with azathioprine, an immunosuppressive drug. Compared to the PBS-treated control group, azathioprine-immunosuppressed ferrets exhibited a longer period of clinical illness, higher virus titers in nasal turbinate, delayed virus clearance, and significantly lower serum neutralization (SN) antibody titers. Taken together, all antiviral drugs tested marginally reduced the overall clinical scores of infected ferrets but did not significantly affect in vivo virus titers. Despite the potential discrepancy of drug efficacies between animals and humans, these preclinical ferret data should be highly informative to future therapeutic treatment of COVID-19 patients.IMPORTANCE The SARS-CoV-2 pandemic continues to spread worldwide, with rapidly increasing numbers of mortalities, placing increasing strain on health care systems. Despite serious public health concerns, no effective vaccines or therapeutics have been approved by regulatory agencies. In this study, we tested the FDA-approved drugs lopinavir-ritonavir, hydroxychloroquine sulfate, and emtricitabine-tenofovir against SARS-CoV-2 infection in a highly susceptible ferret infection model. While most of the drug treatments marginally reduced clinical symptoms, they did not reduce virus titers, with the exception of emtricitabine-tenofovir treatment, which led to diminished virus titers in nasal washes at 8 dpi. Further, the azathioprine-treated immunosuppressed ferrets showed delayed virus clearance and low SN titers, resulting in a prolonged infection. As several FDA-approved or repurposed drugs are being tested as antiviral candidates at clinics without sufficient information, rapid preclinical animal studies should proceed to identify therapeutic drug candidates with strong antiviral potential and high safety prior to a human efficacy trial.
Keywords: COVID-19; antiviral therapeutics; ferrets; immunosuppression; serum neutralization; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Copyright © 2020 Park et al.
Figures


References
-
- WHO. 2020. Coronavirus disease 2019 (COVID-19) situation report 88. WHO, Geneva, Switzerland: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2.... Accessed 17 April 2020.
-
- To KK-W, Tsang OT-Y, Leung W-S, Tam AR, Wu T-C, Lung DC, Yip CC-Y, Cai J-P, Chan JM-C, Chik TS-H, Lau DP-L, Choi CY-C, Chen L-L, Chan W-M, Chan K-H, Ip JD, Ng AC-K, Poon RW-S, Luo C-T, Cheng VC-C, Chan JF-W, Hung IF-N, Chen Z, Chen H, Yuen K-Y. 2020. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 20:565–574. doi:10.1016/S1473-3099(20)30196-1. - DOI - PMC - PubMed
-
- Choy K-T, Wong AY-L, Kaewpreedee P, Sia SF, Chen D, Hui KPY, Chu DKW, Chan MCW, Cheung PP-H, Huang X, Peiris M, Yen H-L. 2020. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res 178:104786. doi:10.1016/j.antiviral.2020.104786. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
