How a tailor achieves the perfect fit
- PMID: 32444419
- PMCID: PMC7247309
- DOI: 10.1074/jbc.H120.013868
How a tailor achieves the perfect fit
Abstract
Most antigenic peptides that bind stably to a major histocompatibility complex (MHC) I molecule for display to the immune system are approximately the same length, thanks in part to the expert trimming done by endoplasmic reticulum aminopeptidases (ERAPs), the final peptidases in the antigen-presentation pathway. An open question is whether ERAPs edit peptides to this optimal length while they are bound to MHC I molecules (using the latter as a pattern of sorts) or by free hand. Mavridis et al. present multiple lines of evidence that this trimming cannot readily occur on MHC I molecules, but rather only in solution, suggesting that ERAPs work alone to tailor the perfect fit for the immunopeptidome.
© 2020 Colbert and Rock.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
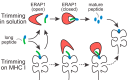
Comment on
-
A systematic re-examination of processing of MHCI-bound antigenic peptide precursors by endoplasmic reticulum aminopeptidase 1.J Biol Chem. 2020 May 22;295(21):7193-7210. doi: 10.1074/jbc.RA120.012976. Epub 2020 Mar 17. J Biol Chem. 2020. PMID: 32184355 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

